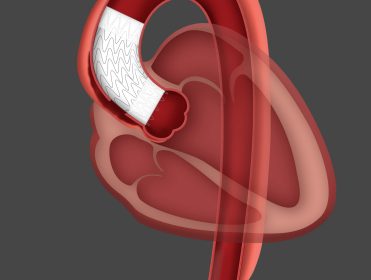
After a decade of clinical use* and more than 50,000 devices sold globally, the GORE® CARDIOFORM Septal Occluder continues a trusted legacy of safely advancing care. FLAGSTAFF, Ariz., Jan. 19, 2022 /PRNewswire/ — W. L. Gore & Associates (Gore) today announced that the GORE® CARDIOFORM Septal Occluder has achieved 10 years of clinical […]
Tag: WL Gore
W. L. Gore & Associates Enhances GORE® VIABAHN® Endoprosthesis Portfolio With Lower Profile Delivery
Improvements to large diameter devices include accessibility through smaller sheaths while providing enhanced visualization by adding radiopaque markers 25 years of continued innovation PUTZBRUNN, Germany, Sept. 22, 2021 /PRNewswire/ — As part of efforts to continuously improve medical solutions for patients with complex vascular disease, W. L. Gore & Associates, Inc. (Gore) announced […]
Physicians In The U.S. Begin Commercial Use Of The GORE® EXCLUDER® Conformable AAA Endoprosthesis With ACTIVE CONTROL System
FLAGSTAFF, Ariz., March 30, 2021 /PRNewswire/ — Today, W. L. Gore & Associates, Inc. (Gore) announces the first use of the FDA approved GORE® EXCLUDER® Conformable AAA Endoprothesis with ACTIVE CONTROL System in cases outside of clinical trials. Cases were successfully performed by Robert Rhee, M.D. with Mahmoud Almadani, M.D., in Brooklyn, New York and by Gustavo Oderich, M.D. with Naveed Saqib, M.D., in Houston, Texas. The GORE […]
W. L. Gore & Associates Enhances GORE® VIABAHN® Endoprosthesis Portfolio With Lower Profile Delivery
Improvements to large diameter devices include accessibility through smaller sheaths while providing enhanced visualization under fluoroscopy FLAGSTAFF, Ariz., Sept. 22, 2020 /PRNewswire/ — As part of efforts to continuously improve medical solutions for patients with complex vascular disease, W. L. Gore & Associates, Inc. (Gore) announced the U.S. launch of the lower profile, large […]
Gore Completes Patient Enrollment in U.S. Pivotal Clinical Study of GORE® CARDIOFORM ASD Occluder
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) has completed enrollment for the pivotal phase of the Gore ASSURED Clinical Study. This investigational device exemption (IDE) trial is researching the new GORE®CARDIOFORM ASD Occluder for the interventional closure of Atrial Septal Defects (ASDs), sized 8 to 35 mm. The […]
Gore Announces Successful Patient Implant of Endovascular Stent Graft for the Ascending Aorta
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced the first implant in conjunction with the Gore ARISE Study of the GORE® Ascending Stent Graft, an investigational device and the only endovascular stent graft specifically designed to treat Type A dissections of the ascending aorta. The successful procedure took place […]
GORE® Molding & Occlusion Balloon for Endovascular Aortic Repair Receives Approval in the United States, Japan, and Europe
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) announced FDA 510(k) clearance, approval from the Japanese Ministry of Health, Labour, and Welfare, and receipt of CE Mark for the innovative GORE® Molding & Occlusion Balloon, a compliant polyurethane balloon catheter designed in close collaboration with clinicians to assist in the expansion […]
Gore Announces First Commercial In-Human Use of GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System for TEVAR in Australia
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced the first patient implant of the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System after being included on the Australian Register of Therapeutic Goods last month in Australia. The first implants were performed by Professor Ian Spark […]
FDA Approves GORE® CARDIOFORM Septal Occluder for PFO Closure to Prevent Recurrent Ischemic Stroke
FLAGSTAFF, Ariz., April 3, 2018 /PRNewswire/ — Following the unprecedented Gore REDUCE Clinical Study conclusion that closure of patent foramen ovale (PFO) can prevent recurrent ischemic strokes, W. L. Gore & Associates, Inc. (Gore) has received approval from the U.S. Food and Drug Administration (FDA) for an expanded indication for its GORE® CARDIOFORM Septal Occluder. The device, already […]
Gore Announces Positive Results from REDUCE Clinical Study for PFO Closure
Study met its primary endpoint with PFO closure in conjunction with antiplatelet therapy, reducing recurrent stroke by > 75% over antiplatelet therapy alone Share: Twitter Linked In Source W. L. Gore Press Release FLAGSTAFF, Ariz. (May 16, 2017) – W. L. Gore & Associates, Inc. (Gore) announces positive results from its […]