American Heart Association consortium advances development of evidence-based health tech solutions
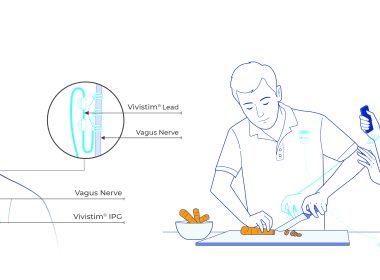
Vivistim is an FDA-approved breakthrough device that works with the brain and body to make stroke rehabilitation therapy and daily activities more effective.
AUSTIN, Texas, Jan. 16, 2026 (GLOBE NEWSWIRE) — MicroTransponder®, Inc., a commercial-stage medical technology company and developer of the breakthrough Vivistim® Paired VNS™ System for chronic stroke recovery, has joined the American Heart Association Center for Health Technology & Innovation’s (the Center) Innovators’ Network. The Center is focused on building and fostering health technology relationships to develop innovative and scalable solutions. The Innovators’ Network is a consortium connecting entrepreneurs, providers, researchers and payers to advance cardiovascular and brain health innovation. Innovators’ Network members also have the opportunity to access the Association’s digital evidence-based scientific guidelines and clinical recommendations as they develop digital healthcare technologies. Members collaborate with the Center in different ways, including building of models for clinical outcome studies, which lowers the significant cost of developing those studies independently, helping connect the science to technology and providing evidence that a digital platform improves healthcare outcomes – a key concern for providers and payers. “The Center aims to advance the rapid, efficient, and effective development of healthcare technology,” said Robert A. Harrington, M.D., FAHA, volunteer past president of the American Heart Association (2019-2020), chair of the American Heart Association’s Health Tech Advisory Group for the Center and the Stephen and Suzanne Weiss Dean of Weill Cornell Medicine and provost for medical affairs of Cornell University. “Joining the Innovators’ Network gives members the opportunity to leverage the consortium and work toward broadening and deepening their engagement in this arena.” “We’re honored to join the American Heart Association’s Innovators’ Network and contribute our growing real-world insights to the Center,” said Richard Foust, MicroTransponder’s president and CEO. “Being part of this consortium strengthens collaboration across the stroke-recovery community and supports efforts to expand access to innovative approaches that could improve outcomes for stroke survivors nationwide.” MicroTransponder, Inc. brings Vivistim Paired VNS Therapy to the Innovators’ Network. The Vivistim System was approved by the FDA for commercial use in 2021 with a Breakthrough Device Designation. It is the first and only implantable solution clinically proven to help chronic ischemic stroke survivors with moderate to severe upper extremity impairments regain 2-3 times greater upper extremity function than intensive rehabilitation alone. The Vivistim System is activated during occupational or physical therapy to pair vagus nerve stimulation with high-repetition, goal-oriented functional activities, which helps enhance neuroplasticity to remodel neural pathways. About MicroTransponderMicroTransponder®, Inc., is a commercial-stage medical device company redefining stroke recovery for survivors living with life-altering motor impairments. The company’s breakthrough Vivistim® Paired VNS™ Therapy is the first FDA-approved implantable solution designed to improve upper limb function in chronic ischemic stroke survivors with moderate to severe upper extremity impairments. Vivistim Paired VNS Therapy combines targeted vagus nerve stimulation with functional movement to promote neuroplasticity and drive meaningful improvements in motor function. For more information, visit Vivistim.com and review safety information at Vivistim.com/safety. MEDIA CONTACT: Kristin Strauderkristin@microtransponder.com A video accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/030fe98a-12b1-4418-8237-4cc7e3120b83 A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/2c22a105-7e71-4ec9-9648-ad95e9545c9f