WESTMINSTER, Colo.–(BUSINESS WIRE)–TriSalus Life Sciences, Inc. (Nasdaq: TLSI) (“TriSalus” or the “Company”), an oncology company integrating novel delivery technology with standard of care therapies to transform treatment for patients with solid tumors, announced today that it will host a virtual key opinion leader (KOL) event on Monday, December 15, 2025 […]
Author: Ken Dropiewski
Study Leverages Vivalink’s Biometrics Data Platform to Uncover Connection Between Seizures and Cardiac Function
Ochsner Health study explores how continuous cardiac monitoring can help assess seizure-related health risks and guide treatment decisions for patients with epilepsy Campbell, Calif. – Dec. 4, 2025 – Vivalink, a leading provider of digital healthcare solutions, is supporting a hospital-based study at Ochsner Health in New Orleans, LA, […]
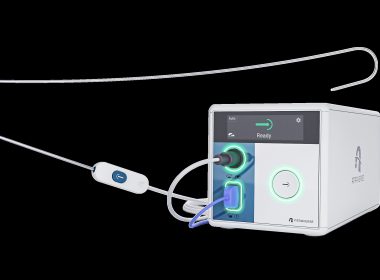
Atraverse Medical Secures FDA 510(k) Clearance for HOTWIRE™ RF Generator & Fully Integrated Transseptal Access System
Atraverse Medical also approaches first 2,000 successful clinical procedures using the company’s innovative HOTWIRE™ radiofrequency (RF) guidewire. [San Diego, CA – December 3, 2025] — Atraverse Medical, a medical device company pioneering next-generation left-heart access technology, has received 510(k) clearance from the U.S. FDA for its fully integrated HOTWIRE™ Transseptal […]
Edwards Lifesciences Reaffirms Strategy for Sustainable, Differentiated Growth at Annual Investor Conference
Innovation with Purpose, Powered by Science, Centered on Patients: Edwards’ Commitment to Drive Value for the Healthcare Ecosystem IRVINE, Calif.–(BUSINESS WIRE)–Edwards Lifesciences (NYSE: EW) will outline its patient-focused strategy and share financial guidance during its annual investor conference. Entering 2026 with momentum for sustainable differentiated growth, Edwards is uniquely positioned with […]
VALVOSOFT®, the First Non-Invasive Treatment for Severe Symptomatic Aortic Stenosis (sSAS) from Cardiawave Receives CE Marking
LEVALLOIS-PERRET, France–(BUSINESS WIRE)–Cardiawave, a pioneering medtech company developing non-invasive ultrasound therapy (NIUT) for aortic stenosis, has received CE Certificate for Valvosoft®, the world’s first non-invasive therapeutic alternative to treat Severe Symptomatic Aortic Stenosis (sSAS), a serious, degenerative and fast-growing disease due to population aging, which remains without a solution for […]
Delcath Systems Announces Publication of 10-Year Single-Center Experience with Percutaneous Hepatic Perfusion in Liver-Dominant Metastatic Uveal Melanoma
QUEENSBURY, N.Y.–(BUSINESS WIRE)–Delcath Systems, Inc. (Nasdaq: DCTH), (“Delcath” or the “Company”) an interventional oncology company focused on the treatment of primary and metastatic liver cancers, today announced the publication of a retrospective study by leading interventional radiologists and oncologists from Asklepios Hospital Barmbek in Hamburg, Germany. The study, titled “Survival […]
Rising Stars in Cardiac Electrophysiology: ABC Announces 2026-2027 Fellowship Recipients
WASHINGTON, Dec. 4, 2025 /PRNewswire/ — The Association of Black Cardiologists (ABC) proudly announces the recipients of its 2026–2027 Cardiac Electrophysiology (EP) Fellowship Support Awards, which recognize outstanding physicians pursuing advanced subspecialty training in cardiac…
Lexeo Therapeutics to Host Virtual Key Opinion Leader Event at the 22nd Global Cardiovascular Clinical Trialists (CVCT) Forum
NEW YORK, Dec. 04, 2025 (GLOBE NEWSWIRE) — Lexeo Therapeutics, Inc. (Nasdaq: LXEO), a clinical stage genetic medicine company dedicated to pioneering novel treatments for cardiovascular diseases, today announced plans to host a virtual event, “A Clinician’s Perspective: Holistic Approach to Managing PKP2-Associated Arrhythmogenic Cardiomyopathy,” at the 22nd Global Cardiovascular Clinical Trialists (CVCT) Forum on Tuesday, December 9th at 3 PM ET. The session will feature a discussion with Dr. Victoria Parikh, M.D., who is Associate Professor of Cardiovascular Medicine at Stanford University and Director of the Stanford Center for Inherited Cardiovascular Disease, and an expert clinician scientist who cares for people living with PKP2-associated arrhythmogenic cardiomyopathy (PKP2-ACM). Analysts and investors can join the live event by registering on the News & Events page on Lexeo’s website. A replay will be archived on the investor section of Lexeo’s website following the event. About Lexeo TherapeuticsLexeo Therapeutics is a New York City-based, clinical stage genetic medicine company dedicated to reshaping heart health by applying pioneering science to fundamentally change how cardiovascular diseases are treated. The Company is advancing a portfolio of therapeutic candidates that take aim at the underlying genetic causes of conditions, including LX2006 in Friedreich ataxia (FA), LX2020 in plakophilin-2 (PKP2) arrhythmogenic cardiomyopathy, and others in devastating diseases with high unmet need. Media Response:Media@lexeotx.com Investor Response:Carlo Tanzi, Ph.D.ctanzi@kendallir.com
Elucid Gains Broad CMS Reimbursement of Coronary Plaque Analysis Using PlaqueIQ™
New CPT I Code, OPPS and MPFS Decisions Recognize Significant Clinical Value Across Care Settings BOSTON–(BUSINESS WIRE)–Elucid has announced new Hospital Outpatient Prospective Payment (OPPS) and Physician Fee Schedule (PFS) decisions from the U.S. Centers for Medicare & Medicaid Services (CMS) that establish significant reimbursement for coronary plaque analysis using PlaqueIQ […]
Picard Medical, Inc. and SynCardia Systems LLC CEO to Present at CSI Focus D-HF 2025 in Frankfurt, Germany
TUCSON, Ariz., Dec. 03, 2025 (GLOBE NEWSWIRE) — Picard Medical, Inc. (NYSE American: PMI) (“Picard” or the “Company”), parent company of SynCardia Systems LLC, maker of the world’s first total artificial heart approved by both the U.S. FDA and Health Canada, today announced that Patrick NJ Schnegelsberg, CEO will attend and present data on the fully implantable Emperor Total Artificial Heart (TAH) at the upcoming CSI Focus D-HF (Device Therapies in Heart Failure) conference, scheduled for December 5th through 6th, 2025 in Frankfurt, Germany.