By Ken Dropkiewski (ken@prime-core.com) While no hard statistics exist as to the exact number of motor vehicle accidents and concurrent heart attacks, the article 5 Facts About the Trend of Heart Attacks While Driving, makes a compelling case for why more people are suffering acute MIs while driving. – An aging population […]
Author: Ken Dropiewski
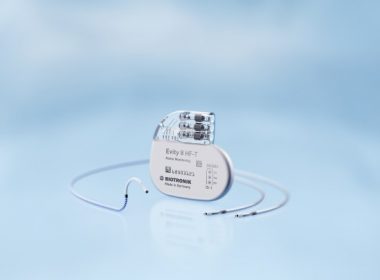
BIOTRONIK’s New Quadripolar CRT-P with More Pacing Options Now Available in Japan
Evity 8 HF-T QP Offers a Host of Functions Including LV VectorOpt, RF Telemetry, MRI AutoDetect and BIOTRONIK Home Monitoring TOKYO, Japan and BERLIN, Germany, July 10, 2017 – BIOTRONIK has announced the launch of its Evity cardiac resynchronization therapy pacemaker (CRT-P) in the Japanese market. It is the company’s highest performing […]
LimFlow Announces First Patient Treated In U.S. Feasibility Study And Commencement Of International Post-Market Study
PARIS–(BUSINESS WIRE)–LimFlow SA today announced enrollment of the first patient in the U.S. feasibility study of the LimFlow Percutaneous Deep Vein Arterialization (pDVA) System, a novel, purely percutaneous device for the treatment of end-stage critical limb ischemia (CLI) when all other revascularization efforts have been exhausted. The first patient was treated […]
Stent coated with Viagra may help prevent blood clots
Stent coated with Viagra may help prevent blood clots By AMERICAN HEART ASSOCIATION NEWS Coating heart stents with the erectile dysfunction drug Viagra, known by the generic name sildenafil, could one day reduce blood clots and re-narrowing of arteries, according to new research. Stents prop open heart arteries to help […]
Medtronic Expands TAVR Access to More Patients With Symptomatic, Severe Aortic Stenosis Upon Intermediate Risk FDA Approval
DUBLIN – July 10, 2017 – Medtronic plc (NYSE: MDT) today announced the expanded U.S. Food and Drug Administration (FDA) approval of the self-expanding CoreValve(TM) Evolut(TM) transcatheter aortic valve replacement (TAVR) platform to include patients with symptomatic severe aortic stenosis who are at an intermediate risk for open-heart surgery. With hemodynamic […]
CryoLife (CRY) Announces Release Date And Teleconference Call Details For 2017 Second Quarter Financial Results
ATLANTA, July 10, 2017 /PRNewswire/ — CryoLife, Inc. (NYSE: CRY), a leading medical device and tissue processing company focused on cardiac surgery, announced today that 2017 second quarter financial results will be released on Monday, July 24, 2017 after the market closes. On Tuesday, July 25, 2017 the Company will hold a teleconference call and live […]
Resverlogix (RVX.TO) Randomizes First Patient In Taiwan/China Portion Of The Phase III BETonMACE Clinical Trial
CALGARY, July 10, 2017 /PRNewswire/ – Resverlogix Corp. (“Resverlogix” or the “Company”) (TSX:RVX) today announced that the first patient has been randomized in Taiwan in the Phase 3 BETonMACE clinical trial with lead drug apabetalone intended for high-risk patients with cardiovascular disease (“CVD”) and type 2 diabetes mellitus (“DM”). On July 8, 2015, Resverlogix and […]
Intact Vascular Release: FDA Approves 6-Month Primary Endpoint For The Tack Endovascular System In Below The Knee Disease
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the U.S. Food and Drug Administration (FDA) approved an Investigational Device Exemption (IDE) supplemental application to modify the primary endpoint in the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial from 12 months to […]
RenalGuard Names Former St. Jude Medical (STJ)-Abiomed (ABMD) Exec as New CEO
MILFORD, MA–(Marketwired – July 10, 2017) – RenalGuard Solutions, Inc. today announced the appointment of Jim Dillon, former St. Jude Medical and Abiomed commercial executive, as Chief Executive Officer. “Jim joins RenalGuard with a proven record of executive leadership related to innovative medical devices that serve the needs of the interventional […]
Onyx DES launching this week in Japan
Medtronic will add Onyx to the Resolute stents available in Japan (Integrity has been selling there since 2012). Resolute Onyx drug-eluting stent launches in Japan JULY 7, 2017 BY SARAH FAULKNER Medtronic (NYSE:MDT) said this week that it’s Resolute Onyx drug-eluting stent will launch in Japan on July 10th. The drug-eluting stent has […]