WAYNE, Pa.–(BUSINESS WIRE)–Cagent Vascular, a developer of next generation technology for vessel dilatation in cardiovascular disease interventions, including the FDA 510(k) cleared Serranator® Alto PTA Serration Balloon Catheter, announces the completion of enrollment in the First-in-Human PRELUDE Study. The purpose of this prospective, single-arm, multicenter feasibility study is to show […]
Author: Ken Dropiewski
OrbusNeich Announces FDA Clearance, Launch of Coronary Dilatation Catheters
HONG KONG, June 6, 2017 /PRNewswire/ — Announcement marks company‘s official entry into the US market OrbusNeich, a global company specializing in the provision of life-changing vascular solutions, has announced the launch of its world-renowned Sapphire PTCA balloon dilatation catheters following their recent 510k clearance by the FDA: the Sapphire™ […]
Avera Names Myles Greenberg, MD, as CEO of Alucent Medical
SIOUX FALLS, S.D.–(BUSINESS WIRE)–Myles D. Greenberg, MD, was named President and CEO of Alucent Medical, Inc., a new company licensed to develop a novel drug/device combination therapy for the treatment of peripheral vascular disease (PVD). The treatment, Natural Vascular Scaffolding (NVS)TM, has recently gained FDA approval to move forward with […]
Edwards SAPIEN 3 Valve Receives FDA Approval For Aortic, Mitral Valve-In-Valve Procedures
IRVINE, Calif., June 5, 2017 — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced it has received U.S. Food and Drug Administration (FDA) approval for aortic and mitral valve-in-valve procedures using the Edwards SAPIEN 3 transcatheter heart valve. […]
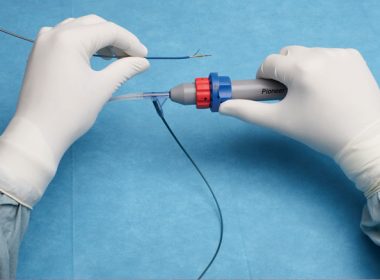
Philips announces the relaunch of its Pioneer Plus catheter, the only re-entry device with intravascular ultrasound guidance
Phillips News Release AMSTERDAM, the Netherlands – Royal Philips (NYSE: PHG AEX: PHIA) today announced the relaunch of its innovative Pioneer Plus catheter, the first and only re-entry device with intravascular ultrasound (IVUS) capabilities and needle deployment designed to assist arterial vessel intervention. IVUS captures images of vessels in the […]
InspireMD Announces Appointment Of Paul Stuka As Chairman Of The Board
TEL AVIV, ISRAEL–(Marketwired – June 02, 2017) – InspireMD, Inc. (NYSE MKT: NSPR) (NYSE MKT: NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced that Paul Stuka, who has served on the Company’s Board of Directors since 2011, […]
Aziyo Continues Building New Breed of Regenerative Medicine Company
SILVER SPRING, Md.–(BUSINESS WIRE)–Aziyo Biologics, Inc. a fully integrated regenerative medicine company, today announced it has completed an asset purchase agreement with CorMatrix� Cardiovascular, Inc. for the purchase of all CorMatrix commercial assets and related intellectual property. With the acquisition, Aziyo will: Add 5 commercially marketed products Double topline revenue Add […]
Medical Device Company TandemLife Set to Simplify Life Support
PITTSBURGH–(BUSINESS WIRE)–TandemLife announced today the commercial release of its TandemLife Priming Tray, a new product designed to simplify and speed up the process of putting critically-ill patients on extracorporeal life support, providing healthcare professionals with a vital window of opportunity to accurately assess a patient’s condition and provide appropriate care. […]
RA Medical’ DABRA Begins First Commercial, FDA-Cleared, In-Patient Use
NEW ORLEANS, La.–(BUSINESS WIRE)–Ra Medical Systems, makers of cardiovascular and dermatology catheters and excimer lasers, today announced the commercial launch in the United States of the Company’s groundbreaking DABRA™ System for the treatment of Peripheral Artery Disease. This follows FDA clearance earlier this week. Dr. Athar Ansari of the California […]
Somahlution Announces Patient Enrollment In Duragraft CABG European Registry
JUPITER, Fla., Jan. 4, 2017 /PRNewswire/ — Somahlution, a global biotechnology company developing products to reduce the burden of ischemia reperfusion injury in tissue grafting, organ transplant and other surgical indications, today announced enrollment of the first subject in their DuraGraft CABG European Registry. The registry will evaluate the company’s flagship […]