Building on FDA clearances, enterprise AI leadership, and global expansion, RapidAI and AWS are advancing secure, scalable AI that transforms imaging AI CHICAGO–(BUSINESS WIRE)–RapidAI, the pioneer of deep clinical AI and global leader in enterprise imaging, and Amazon Web Services (AWS), today announced a strengthened working relationship to accelerate the […]
Author: Ken Dropiewski
Mindray Unveils a Breakthrough in Ultra Premium Ultrasound at RSNA 2025: The Resona A20
Revolutionizing radiology with cutting-edge imaging, smart automation, and effortless control — the Resona A20 is built to elevate diagnostic confidence like never before. MAHWAH, N.J., Dec. 1, 2025 /PRNewswire/ — Mindray, a global leader in healthcare technologies and solutions…
MMI Announces CPT Code and CMS Reimbursement for Robotic Lymphatic Surgery, Unlocking Market Access and Growth Potential
MMI, today announced that the American Medical Association (AMA) has issued a new Current Procedural Terminology (CPT®) code for lymphovenous bypass.
Cadrenal Therapeutics Appoints Dr. Lee Golden to Board of Directors
PONTE VEDRA, Fla., Dec. 01, 2025 (GLOBE NEWSWIRE) — Cadrenal Therapeutics, Inc. (Nasdaq: CVKD), a biopharmaceutical company developing transformative therapeutics to overcome current gaps in anticoagulation therapy, today announced the appointment of Lee Scott Golden, M.D., to its Board of Directors, effective immediately. Dr. Golden will serve as an independent director. Dr. Golden currently serves as Executive Vice President and Chief Medical Officer at PTC Therapeutics, Inc. (Nasdaq: PTCT), where he leads global clinical development across a broad rare disease pipeline. Before joining PTC, Dr. Golden served as the Chief Medical Officer at Espero BioPharma, Inc., a development-stage cardiovascular pharmaceutical company focused on developing drugs for unmet needs in thrombosis and cardiac rhythm control, and as Chief Medical Officer at Gemphire Therapeutics, Inc. He also serves as Chairman of the Advisory Board for Coagulation Sciences LLC. Previously, Dr. Golden held senior roles at Pfizer, Actelion, Eisai, Mesoblast, and others, with a long-standing focus on cardiovascular and hematologic drug development. “We are delighted to welcome Dr. Golden to our Board,” said Quang X. Pham, Chairman and Chief Executive Officer of Cadrenal Therapeutics. “Lee’s deep experience in late-stage clinical development, particularly in cardiovascular medicine and anticoagulation, is highly aligned with our mission to deliver safer, more predictable anticoagulant options for patients with significant unmet needs. His track record in guiding therapies through clinical development and regulatory pathways will be invaluable as we continue to advance tecarfarin and our broader pipeline.” “Cadrenal Therapeutics is working in an area of high clinical importance, where better anticoagulation options could meaningfully impact outcomes for patients with complex cardiovascular conditions,” said Dr. Golden. “I look forward to working with the Board and the leadership team to help guide the Company’s strategy and clinical programs as we seek to bring differentiated therapies to patients and create value for shareholders.” Dr. Golden has more than 25 years of industry experience, with increasing responsibilities, managing global, cross-functional teams responsible for creating and deploying strategic and clinical development plans. He has extensive experience across multiple therapeutic areas and with orphan diseases. Dr. Golden received a B.S. from the University of Michigan and an MD from New York University School of Medicine, where he also completed his Internal Medicine residency. He then completed Fellowships in Cardiology at the University of Miami and Interventional Cardiology at George Washington University Hospital, where he also served as an adjunct instructor. About Cadrenal Therapeutics, Inc. Cadrenal Therapeutics, Inc. is a biopharmaceutical company with a mission to develop novel and differentiated biopharmaceutical products that bridge critical gaps in current acute and chronic anticoagulant therapy. We bridge these gaps by developing novel and differentiated anticoagulants, or blood thinners, designed to provide greater predictability, increased stability, more precise control, and fewer bleeding complications. We currently have two clinical-stage assets: tecarfarin, an oral vitamin K antagonist (VKA) for chronic use, and frunexian, a parenteral small-molecule Factor XIa antagonist for use in acute hospital settings. By targeting underserved patient populations and advancing therapies designed for both chronic and acute use, we aim to reshape standards of care in anticoagulation. For more information, visit https://www.cadrenal.com/ and connect with the Company on LinkedIn. Safe Harbor Any statements in this press release about future expectations, plans, and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements.” The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These statements include statements regarding developing transformative therapeutics to overcome current gaps in anticoagulation therapy, the expected contribution of Dr. Golden and continuing to advance tecarfarin and the Company’s broader pipeline. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the ability to develop transformative therapeutics to overcome current gaps in anticoagulation therapy, Dr. Golden’s ability to help guide the Company’s strategy and clinical programs, the ability to successfully complete clinical trials on time and achieve desired results and benefits as expected and the other risk factors described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, and the Company’s subsequent filings with the Securities and Exchange Commission, including subsequent periodic reports on Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. Any forward-looking statements contained in this press release speak only as of the date hereof and, except as required by federal securities laws, the Company specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events, or otherwise. For more information, please contact: Lytham Partners, LLCRobert Blum, Managing Partner602-889-9700CVKD@lythampartners.com
MannKind Announces U.S. FDA Accepts for Review its Supplemental New Drug Application (sNDA) of FUROSCIX ReadyFlow™ Autoinjector for the Treatment of Edema in Adults with Chronic Heart Failure or Chronic Kidney Disease
MannKind Announces U.S. FDA Accepts for Review its sNDA of FUROSCIX ReadyFlow™ Autoinjector for the Treatment of Edema in adults with CHF or CKD
Rad AI Unveils Next-Generation Speech Recognition That Redefines Radiology Reporting
Breakthrough technology delivers faster, more accurate reports through proprietary multi-model AI and advanced language understanding Builds on Rad AI’s broader reporting innovations, including its RSNA Ventures collaboration, to advance the next generation of radiology efficiency…
Catheter Precision Announces Launch of LockeT in Switzerland
Fort Mill, SC, Dec. 01, 2025 (GLOBE NEWSWIRE) — Catheter Precision, Inc. (VTAK – NYSE/American), a US based medical device company focused on developing technologically advanced products for the cardiac electrophysiology market today announced the successful launch of its LockeT suture retention device in Switzerland. The first clinical cases were performed at Spitalzentrum Biel, led by PD Dr. Rainer Zbinden, with excellent procedural outcomes and positive feedback from both the clinical and nursing teams. This milestone follows the company’s recent strategic distribution agreement with FuMedica AG, a premier Swiss medical device distributor with a strong presence in cardiovascular and interventional medicine. The partnership enables the introduction of LockeT to hospitals and clinics across Switzerland, further expanding Catheter Precision’s European footprint. Fatih Ayoglu, Sales Manager EMEA & APAC at Catheter Precision, said, “We’ve recently partnered with FuMedica to distribute LockeT in Switzerland, and it’s already exciting to see the first cases roll out so quickly. LockeT is intuitive and easy to use—it reduces groin management costs and supports same-day discharge. With over 10,000 LockeTs shipped globally, we’re growing fast and working hard with our partners, one patient at a time.” About LockeTCatheter Precision’s LockeT is a suture retention device intended to assist in wound closure after percutaneous venous punctures. LockeT is a Class 1 device registered with the FDA and has received CE Mark approval. About Catheter PrecisionCatheter Precision is an innovative U.S.-based medical device company bringing new solutions to market to improve the treatment of cardiac arrhythmias. It is focused on developing groundbreaking technology for electrophysiology procedures by collaborating with physicians and continuously advancing its products. Cautionary Note Regarding Forward-Looking StatementsStatements in this press release may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to substantial risk and uncertainties. Forward-looking statements can be identified by words such as “believe,” “anticipate,” “may,” “might,” “can,” “could,” “continue,” “depends,” “expect,” “expand,” “forecast,” “intend,” “predict,” “plan,” “rely,” “should,” “will,” “may,” “seek,” or the negative of these terms and other similar expressions, although not all forward-looking statements contain these words. These forward-looking statements include, but are not limited to, statements regarding product evaluations at the hospital, and that the purchase order indicates that the hospital and its staff see the value and benefits that LockeT can bring and expectations regarding LockeT evaluations in the coming weeks. The Company’s expectations and beliefs regarding these matters may not materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks and changes in circumstances, including but not limited to risks and uncertainties included under the caption “Risk Factors” in the Company’s Form 10-K filed with the SEC and available at www.sec.gov. The forward-looking statements included in this communication are made only as of the date hereof. The Company assumes no obligation and does not intend to update these forward-looking statements, except as required by law. # # # CONTACT: CONTACTS:
Investor Relations
973-691-2000
IR@catheterprecision.com
Kestra Medical Technologies, Ltd. Announces Primary Public Offering of Common Shares
KIRKLAND, Wash., Dec. 01, 2025 (GLOBE NEWSWIRE) — Kestra Medical Technologies, Ltd. (Nasdaq: KMTS), a wearable medical device and digital healthcare company, today announced an underwritten public offering of 5,500,000 common shares. Kestra is offering these shares pursuant to a registration statement on Form S-1 filed with the Securities and Exchange Commission (“SEC”). Kestra also intends to grant the underwriters a 30-day option to purchase up to an additional 825,000 common shares at the public offering price, less underwriting discounts and commissions. The proposed offering is subject to market and other conditions, and there can be no assurances as to whether or when the offering may be completed or as to the actual size or terms of the offering. Kestra intends to use the proceeds to support sales and marketing activities, to drive ongoing commercialization, to further fund our research and development and clinical studies and for working capital and general corporate purposes. All of the common shares are being offered by Kestra. BofA Securities, Piper Sandler, J.P. Morgan, Goldman Sachs & Co. LLC and Wells Fargo Securities are acting as bookrunners for the proposed offering. The proposed offering will be made only by means of a prospectus. Copies of the preliminary prospectus, when available, may be obtained from BofA Securities, Attention: Prospectus Department, NC1-022-02-25, 201 North Tryon Street, Charlotte, NC 28255-0001, or by email at dg.prospectus_requests@bofa.com; Piper Sandler, 350 North 5th Street, Suite 1000, Minneapolis, MN 55401, Attention: Prospectus Department, by telephone at 800-747-3924 or by email at prospectus@psc.com; J.P. Morgan Securities LLC, c/o Broadridge Financial Solutions, 1155 Long Island Avenue, Edgewood, NY 11717, or by email at prospectus-eq_fi@jpmchase.com and postsalemanualrequests@broadridge.com; or Goldman Sachs & Co. LLC, Attention: Prospectus Department, 200 West Street, New York, NY 10282, by telephone at (866) 471-2526 or by email at prospectus-ny@ny.email.gs.com. A registration statement relating to these securities has been filed with the SEC but has not yet become effective. These securities may not be sold, nor may offers to buy be accepted, prior to the time the registration statement becomes effective. This press release shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Cautionary Statements Regarding Forward-Looking Information Except where otherwise noted, the information contained in this press release is as of December 1, 2025. This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about, among other topics, Kestra’s expectations regarding the consummation, timing and size of the offering and the grant of the option to purchase additional shares to the underwriters. Statements in this press release that express a belief, expectation or intention, as well as those that are not based on historical fact, as forward-looking statements. Given their forward-looking nature, these statements involve substantial risks, uncertainties and potentially inaccurate assumptions, and we cannot ensure that any outcome expressed in these forward-looking statements will be realized in whole or in part. You can identify these statements by the fact that they use future dates or use words such as “will,” “may,” “could,” “likely,” “ongoing,” “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” “assume,” “target,” “forecast,” “guidance,” “goal,” “objective,” “aim,” “seek,” “potential,” “hope” and other words and terms of similar meaning. Among the factors that could cause actual results to differ materially from those currently anticipated include risks and uncertainties related to market conditions, satisfaction of customary closing conditions related to the offering and other risks and uncertainties described under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the fiscal year ended April 30, 2025 filed with the SEC on July 17, 2025, and in other periodic reports filed by the Company with the SEC. These filings, when made, are available on the Investor Relations section of our website and on the SEC’s website at https://sec.gov/. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. About Kestra Kestra Medical Technologies, Ltd. is a commercial-stage wearable medical device and digital healthcare company focused on transforming patient outcomes in cardiovascular disease using monitoring and therapeutic intervention technologies that are intuitive, intelligent, and connected. CONTACT: Investor contact
Neil Bhalodkar
neil.bhalodkar@kestramedical.com
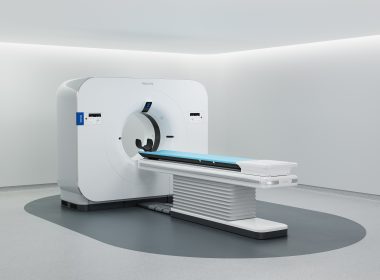
Philips launches Verida, world’s first detector-based spectral CT powered by breakthrough AI, to advance diagnostic precision
Philips Verida spectral CT system, angle view
Philips Verida spectral CT system, angle view
Philips Verida spectral CT system, straight-on view
Philips Verida spectral CT system, straight-on view
November 30, 2025 Philips pioneered detector-based spectral CT, which has been widely adopted in clinical routine exams across anatomies, supported by over 800 peer-reviewed publications [1]CE-marked, 510k pending Verida CT [2] integrates AI across the imaging chain, providing superb image quality while accelerating workflow and reducing dose [3, 4] Amsterdam, the Netherlands and Chicago, USA – At RSNA 2025, Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Verida, the world’s first detector-based spectral CT fully powered by AI. This marks a transformative milestone in CT, with AI optimizing the entire imaging chain – lowering system noise, elevating image quality, and accelerating clinical workflow. With over 800 global installations and supported by over 800 peer-reviewed publications, Philips’ spectral CT uses PACS-native delivery and has been fully embedded into clinical workflow. Spectral CT measures how tissues absorb different x-ray energy levels, enabling differentiation of materials that appear identical on conventional CT. Philips has pioneered detector-based spectral CT, delivering multiple spectral results from a single scan with no tradeoffs in performance or scan time. Now, by integrating AI across the imaging chain, from acquisition to reconstruction, Philips Verida generates industry-leading, superior spectral image quality with minimal noise, in addition to high-definition conventional images. With its full AI capabilities, Verida can achieve dramatic dose reduction [5] without compromising image quality and reduce energy consumption by up to 45% [6]. “The clinical benefits of Verida will fundamentally change my approach to cardiac imaging,” said Prof. Eliseo Vañó Galván, cardiovascular radiologist, Chairman of the CT & MR Department at Hospital Nuestra. Sra. Del Rosario, Madrid, Spain. “With more comprehensive insights in every cardiac CT, I plan to make spectral imaging routine for all patients – building toward a fully spectral CT department. We evaluated many systems, including photon-counting CT, but chose Philips because it delivers the precision we need in a streamlined, easy-to-use platform. The result is greater diagnostic confidence and the potential to reduce the need for invasive angiograms – not just in cardiology, but across other clinical areas as well [7].” Verida reconstructs 145 images per second, so entire exams automatically appear in less than 30 seconds – 2× faster than before and enabling up to 270 exams every day [8]. Building on Philips’ proprietary Spectral Precise Image technology – a deep learning AI reconstruction engine combined with advanced spectral imaging – and its third-generation Nano-panel Precise dual-layer detector with intrinsic noise reduction optimized for AI, Verida is designed to deliver faster, more dose-efficient spectral reconstructions. This enables clinicians to access rich spectral information from a single scan. “Combining the latest advances in our proven spectral CT technology with AI, our flagship Verida CT system is designed to set a new standard in superior image quality and accelerated scans which are fully embedded in the radiology workflow, all to help clinicians detect and characterize disease earlier, reduce variability in diagnoses, and support efficient treatment pathways – in a single scan,” said Dan Xu, Business Leader of CT at Philips. “While photon-counting CT adds complexity, is yet to move from the research arena into clinical practice, Philips spectral CT has been a clinical workhorse for more than a decade and delivers comparable or better clinical outcomes, standing up to the most demanding throughput and at significantly lower total cost of ownership”. Verida extends Philips’ software-defined CT approach, pairing AI-driven spectral precision to advance both clinical and operational outcomes. Built for high-demand environments, it streamlines workflows, reduces repeat scans, and delivers consistently sharp imaging across all care pathways. Philips is debuting Verida at RSNA 2025, with availability in select markets beginning in 2026 [9]. [1] Data on file. [2] CE marked and 510(k) pending. Not currently available for sale in the US.[3] Andersen MB et al. Impact of spectral body imaging in patients suspected for occult cancer: a prospective study of 503 patients. Eur Radiol2020. doi.org/10.1007/s00330-020-06878-7[4] Andersen MB et al. Economic impact of spectral body imaging in the diagnosis of patients suspected of occult cancer. Insights into Imaging 2021. doi.org/10.1186/s13244-021-01116-0. Results of customer testimonies are not predictive of results in other cases, where results may vary.[5] Dose reduction assessments were performed using reference body protocol. In clinical practice, the use of Spectral Precise Image may reduce CT patient dose depending on the clinical task, patient size, and anatomical location. A consultation with a radiologist and a physicist should be made to determine the appropriate dose to obtain diagnostic image quality for the particular clinical task. [6] Based on Axial Body 3D Scan with 80% dose reduction. Energy savings for system preparation is not included.[7] Spectral CT Verida Premium up to 270 (4 CIRS config) exams a day (16 hours dual shift working day) meeting the needs of radiology departments with extended work hours and very high patient throughput.[8] The statements of the clinician reflect independent opinion and are not intended to imply product performance prior to regulatory clearance.[9] Pending regulatory clearance. For further information, please contact: Anna HogrebePhilips Global External RelationsTel.: +1 416 270 6757E-mail: anna.hogrebe@philips.com About Royal Philips Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home.Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2024 sales of EUR 18 billion and employs approximately 67,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachments
Philips Verida spectral CT system, angle view
Philips Verida spectral CT system, straight-on view
Orchestra BioMed Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4)
NEW HOPE, Pa., Nov. 26, 2025 (GLOBE NEWSWIRE) — Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO) (“Orchestra BioMed” or the “Company”), a biomedical company accelerating high-impact technologies to patients through strategic partnerships with market-leading global medical device companies, reported today that, on November 24, 2025, the Compensation Committee of the Orchestra BioMed Board of Directors granted stock options to purchase an aggregate of 151,250 shares of the Company’s common stock to 12 newly hired employees. The awards were granted pursuant to the Orchestra Biomed Holdings, Inc. 2025 New Hire Inducement Plan as an inducement material to each new employee entering employment with Orchestra Biomed, in accordance with Nasdaq Listing Rule 5635(c)(4). Twenty-five percent of the stock options granted to each new employee will vest on the first anniversary of the date such employee commenced employment with the Company, with the remainder vesting ratably each quarter over the following three-year period. Orchestra Biomed is providing this information in accordance with Nasdaq Listing Rule 5635(c)(4). About Orchestra BioMedOrchestra BioMed is a biomedical innovation company accelerating high-impact technologies to patients through strategic collaborations with market-leading global medical device companies. The Company’s two flagship product candidates – Atrioventricular Interval Modulation (AVIM) Therapy and Virtue® Sirolimus AngioInfusion™ Balloon (Virtue SAB) – are currently undergoing pivotal clinical trials for their lead indications, each representing multi-billion-dollar annual global market opportunities. AVIM Therapy is a bioelectronic treatment for hypertension, the leading risk factor for death worldwide, and is designed to be delivered as a firmware upgrade to a pacemaker and achieve immediate, substantial and sustained reductions in blood pressure in patients with hypertensive heart disease. The Company has a strategic collaboration with Medtronic, one of the largest medical device companies in the world, for the development and commercialization of AVIM Therapy for the treatment of uncontrolled hypertension in pacemaker-indicated patients. AVIM Therapy has FDA Breakthrough Device Designation for these patients, as well as an estimated 7.7 million total patients in the U.S. with uncontrolled hypertension despite medical therapy and increased cardiovascular risk. Virtue SAB is a highly differentiated, first-of-its-kind drug delivery angioplasty balloon system designed to deliver a proprietary extended-release formulation of sirolimus, SirolimusEFR™, for the treatment of atherosclerotic artery disease, the leading cause of mortality worldwide. Virtue SAB has been granted Breakthrough Device Designation by the FDA for the treatment of coronary ISR, coronary small vessel disease and below-the-knee peripheral artery disease. For further information about Orchestra BioMed, please visit www.orchestrabiomed.com, and follow us on LinkedIn. Investor Contact:Silas NewcombOrchestra BioMedSnewcomb@orchestrabiomed.com Media Contact:Kelsey Kirk-EllisOrchestra BioMedkkirkellis@orchestrabiomed.com