RESEARCH TRIANGLE PARK, N.C.–(BUSINESS WIRE)–TransEnterix, Inc. (NYSE American:TRXC), a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced that Brett Farabaugh has joined as interim Chief Financial Officer, effective January 2, 2020, replacing Joe Slattery who retired December […]
Author: Ken Dropiewski
Neovasc Announces $10 Million Registered Direct Offering Priced At-The-Market
Vancouver, Canada, Jan. 02, 2020 (GLOBE NEWSWIRE) — via NEWMEDIAWIRE — Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN / TSX: NVCN) announced today that it has entered into definitive agreements with certain institutional investors for the sale of an aggregate of 2,418,322 series A units (“Series A Units”) and series […]
VASCEPA® Approved in Canada to Reduce the Risk of Cardiovascular Events
VASCEPA becomes the first and only Health Canada approved medication for reducing cardiovascular risk beyond cholesterol lowering therapy in the studied high-risk patients approved for treatment Commercial launch expected in mid-February 2020 by Amarin’s commercial partner for Canada VASCEPA approval was supported by clinical results from the REDUCE-IT® trial which included […]
Neovasc Submits Premarket Approval Application to FDA for Neovasc Reducer™
VANCOUVER, Dec. 31, 2019 (GLOBE NEWSWIRE) — via NEWMEDIAWIRE — Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced the submission of a Premarket […]
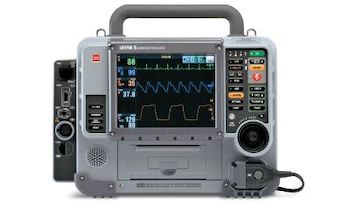
Stryker launches voluntary field action for specific units of the LIFEPAK® 15 monitor/defibrillator
KALAMAZOO, Michigan, USA, Dec. 27, 2019 /PRNewswire/ — Stryker announced today that the company is launching a voluntary field action on specific units of the LIFEPAK 15 monitor/defibrillators. The company is notifying a population of LIFEPAK 15 customers of an issue that may cause their devices to fail to deliver a defibrillation […]
Microbot Medical Inc. Announces $9.59 Million Registered Direct Offering Priced At-the-Market under Nasdaq Rules
HINGHAM, Mass., Dec. 26, 2019 (GLOBE NEWSWIRE) — Microbot Medical Inc. (Nasdaq: MBOT), a medical device company specializing in the design and development of transformational micro-robotic medical technologies, today announced that it has entered into definitive agreements with certain institutional investors for the purchase in a registered direct offering of 912,858 shares […]
Titan Medical Announces New Common Share Purchase Agreement With Aspire Capital Fund of up to US$35 Million
TORONTO–(BUSINESS WIRE)–Titan Medical Inc. (“Titan” or the “Company”) (TSX: TMD) (Nasdaq: TMDI), a medical device company focused on the design, development and commercialization of a robotic surgical system for application in minimally invasive surgery (“MIS”), announces that it has entered into a Common Share Purchase Agreement (the “Agreement”) with Aspire […]
iRhythm Technologies Files Quarterly Report on Form 10-Q for Third Quarter 2019 and Updates Guidance for Full Year 2019
SAN FRANCISCO, Dec. 23, 2019 (GLOBE NEWSWIRE) — iRhythm Technologies, Inc. (NASDAQ: IRTC), a leading digital health care solutions company focused on the advancement of cardiac care, announced the filing of its Quarterly Report on Form 10-Q for the period ended September 30, 2019 with the Securities and Exchange Commission. […]
CryoLife Receives FDA Authorization to Commence PROACT Xa Clinical Trial
Study Designed to Evaluate the Use of Apixaban in On-X Aortic Valve Patients ATLANTA, Dec. 23, 2019 /PRNewswire/ — CryoLife, Inc. (NYSE: CRY), a leading cardiac and vascular surgery company focused on aortic disease, announced today that it has received authorization from the U.S. Food and Drug Administration (FDA) pursuant to an Investigational New […]
Correvio Receives Complete Response Letter From U.S. FDA for Brinavess and Provides Update on Recent Events
NASDAQ: CORV TSX: CORV VANCOUVER, Dec. 24, 2019 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a specialty pharmaceutical company focused on commercializing hospital drugs, today announced it has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) regarding the New Drug Application (NDA) for Brinavess™ (vernakalant IV), an anti-arrhythmic […]