PALO ALTO, Calif., Nov. 03, 2025 (GLOBE NEWSWIRE) — BridgeBio Pharma, Inc. (Nasdaq: BBIO) (“BridgeBio” or the “Company”), a new type of biopharmaceutical company focused on genetic diseases, announced today that ten moderated digital posters will be shared at the American Heart Association (AHA) Scientific Sessions 2025, taking place in New Orleans, LA from November 7 – 10, 2025. Moderated Digital Posters: Acoramidis Effect on All-Cause Mortality in Patients with p.V142I (V122I) Variant ATTR-CM: Findings from the ATTRibute-CM StudyPresenter: Marianna Fontana, M.D., University College London, UKDate/time: Saturday, November 8 at 3:15 pm CT Acoramidis Reduces All-Cause Mortality and First Cardiovascular Hospitalization in Patients with Variant Transthyretin Amyloid Cardiomyopathy: Results from the ATTRibute-CM StudyPresenter: Prem Soman, M.D., Ph.D., University of Pittsburgh School of Medicine, U.S.Date/time: Saturday, November 8 at 9:15 am CT Acoramidis Reduces All-Cause Mortality and Cardiovascular-Related Hospitalizations Through Month 42 in Transthyretin Amyloid Cardiomyopathy Across All Pre-specified Patient SubgroupsPresenter: Lily Stern, M.D., Cedars-Sinai Heart Institute, U.S.Date/time: Saturday, November 8 at 3:15 pm CT Acoramidis Lowers NT-proBNP in a Larger Proportion of ATTRibute-CM Study Participants with Transthyretin Amyloid Cardiomyopathy Compared with Placebo, Independent of Atrial Fibrillation StatusPresenter: Mathew Maurer, M.D., Columbia University Irving Medical Center, U.S.Date/time: Saturday, November 8 at 9:15 am CT Demographic Disparities in Tafamidis Treatment and Clinical Outcomes Across the United StatesPresenter: Nicole Cyrille-Superville, M.D., Atrium Health Sanger Heart & Vascular Institute Kenilworth, Charlotte, NC, U.S.Date/time: Saturday, November 8 at 12:15 pm CT Geographic Disparities in Transthyretin Amyloid Cardiomyopathy Prevalence in United States VeteransPresenter: Sandesh Dev, M.D., Southern Arizona VA Health Care System, U.S.Date/time: Saturday, November 8 at 3:15 pm CT Serum Transthyretin Levels at Day 28 are Associated with Cardiovascular Outcomes: Insights from the ATTRibute-CM StudyPresenter: Nitasha Sarswat, M.D., UChicago Medicine, U.S.Date/time: Sunday, November 9 at 3:15 pm CT Acoramidis Improved Clinical Outcomes, Function, Quality of Life and NT-proBNP in Patients with Transthyretin Amyloid Cardiomyopathy Regardless of Atrial Fibrillation Status at BaselinePresenter: Richard Cheng, M.D., University of Washington, Seattle, WA, U.S.Date/time: Sunday, November 9 at 3:15 pm CT Acoramidis Reduces the Risk of All-Cause Mortality and Cardiovascular-Related Hospitalization Compared with Placebo in Participants with Transthyretin Amyloid Cardiomyopathy and Early-Stage Heart Failure Regardless of Atrial Fibrillation History: Insights from ATTRibute-CM Presenter: Ronald Witteles, M.D., Stanford University School of Medicine, U.S.Date/time: Saturday, November 8 at 3:15 pm CT Vutrisiran Healthcare Resource Utilization, Costs, Discontinuation, and Mortality: A Retrospective Database Analysis Presenter: Nicole Bart, M.D., Ph.D., St Vincent’s Hospital Sydney, AUDate/time: Monday, November 10 at 10:45 am CT About Attruby™ (acoramidis) INDICATIONAttruby is a transthyretin stabilizer indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization. IMPORTANT SAFETY INFORMATION Adverse ReactionsDiarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were reported in patients treated with Attruby versus placebo, respectively. The majority of these adverse reactions were mild and resolved without drug discontinuation. Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively). About BridgeBioBridgeBio Pharma (BridgeBio; Nasdaq: BBIO) is a new type of biopharmaceutical company founded to discover, create, test, and deliver transformative medicines to treat patients who suffer from genetic diseases. BridgeBio’s pipeline of development programs ranges from early science to advanced clinical trials. BridgeBio was founded in 2015 and its team of experienced drug discoverers, developers and innovators are committed to applying advances in genetic medicine to help patients as quickly as possible. For more information visit bridgebio.com and follow us on LinkedIn, X, Facebook, Instagram, and YouTube. BridgeBio Media Contact:Bubba Murarka, Executive Vice President, Corporate Developmentcontact@bridgebio.com (650)-789-8220 BridgeBio Investor Contact:Chinmay Shukla, Senior Vice President, Strategic Financeir@bridgebio.com
Coronary/Structural Heart
FDA Approves Anteris’s DurAVR® THV Global Pivotal Trial (the “PARADIGM Trial”)
PARADIGM: A Prospective rAndomized tRial Assessing the safety and effectiveness of the DurAVR® bIomimetic valve designed for physioloGic flow compared to CoMmercial TAVR devices MINNEAPOLIS and BRISBANE, Australia, Nov. 03, 2025 (GLOBE NEWSWIRE) — Anteris announced today it has received U.S. Food and Drug Administration (FDA) approval to initiate PARADIGM, its global Investigational Device Exemption (IDE) clinical trial which is designed to evaluate the DurAVR® Transcatheter Heart Valve (THV) in patients with severe calcific aortic stenosis and to support a future PMA* submission. “We are extremely pleased to receive FDA approval for the PARADIGM Trial, which allows us to commence patient recruitment in the United States**. This milestone, together with the recent launch of the trial and first patients treated in Denmark, represents a significant achievement and a key step forward in advancing this life-saving technology worldwide for patients living with aortic stenosis, a debilitating and progressive condition,” commented Vice Chairman and CEO, Wayne Paterson. The PARADIGM Trial is co-chaired by Dr. Michael J. Reardon, Allison Family Distinguished Chair of Cardiovascular Research and Professor of Cardiothoracic Surgery at the Houston Methodist Hospital, Texas and Professor Stephan Windecker, Chairman of the Department of Cardiology at Bern University Hospital, Switzerland. About the PARADIGM Trial The PARADIGM Trial is a prospective randomized controlled trial (RCT) which will evaluate the safety and effectiveness of the DurAVR® THV compared to commercially available transcatheter aortic valve replacements (TAVRs). This head-to-head study will enroll approximately 1000 patients across the Unites States, Europe and Canada in the ‘All Comers Randomized Cohort’ with 1:1 randomization of patients who will receive either the DurAVR® THV or TAVR using commercially available and approved THVs. The PARADIGM Trial will assess non-inferiority on a primary composite endpoint of all-cause mortality, all stroke and cardiovascular hospitalization at one year post procedure. The PARADIGM Trial is designed to provide the robust clinical evidence required to support an application to the FDA for Premarket Approval in the United States, with CE Mark approval anticipated to progress in parallel to the PMA. For further information, please refer to ClinicalTrials.gov (ClinicaTrials.gov ID NCT07194265). *A Premarket Approval (PMA) application requires a high level of clinical evidence to demonstrate reasonable assurance of safety and effectiveness for the intended use. Randomized controlled trials are generally considered Level 1 evidence, the highest level for determining the effectiveness of interventions in evidence-based medicine given RCTs minimize bias and allow a clear comparison between treatment groups. **Subject to Institutional Review Board (IRB) approval About Anteris Anteris Technologies Global Corp. (NASDAQ: AVR, ASX: AVR) is a global structural heart company committed to designing, developing, and commercializing cutting-edge medical devices to restore healthy heart function. Founded in Australia, with a significant presence in Minneapolis, USA, Anteris is a science-driven company with an experienced team of multidisciplinary professionals delivering restorative solutions to structural heart disease patients. Anteris’ lead product, the DurAVR® Transcatheter Heart Valve (THV), was designed in partnership with the world’s leading interventional cardiologists and cardiac surgeons to treat aortic stenosis – a potentially life-threatening condition resulting from the narrowing of the aortic valve. The balloon-expandable DurAVR® THV is the first biomimetic valve, which is shaped to mimic the performance of a healthy human aortic valve and aims to replicate normal aortic blood flow. DurAVR® THV is made using a single piece of molded ADAPT® tissue, Anteris’ patented anti-calcification tissue technology. ADAPT® tissue, which is FDA-cleared, has been used clinically for over 10 years and distributed for use in over 55,000 patients worldwide. The DurAVR® THV System is comprised of the DurAVR® valve, the ADAPT® tissue, and the balloon-expandable ComASUR® Delivery System. Forward-Looking Statements This announcement contains forward-looking statements including statements regarding the intent for the PARADIGM Trial. Forward-looking statements include all statements that are not historical facts. Forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “budget,” “target,” “aim,” “strategy,” “plan,” “guidance,” “outlook,” “may,” “should,” “could,” “will,” “would,” “will be,” “will continue,” “will likely result” and similar expressions, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described under “Risk Factors” in Anteris’ Annual Report on Form 10-K for the fiscal period ended December 31, 2024 that was filed with the SEC and ASX. Readers are cautioned not to put undue reliance on forward-looking statements, and except as required by law, neither ATL or Anteris assume any obligation to update any of these forward-looking statements to conform these statements to actual results or revised expectations. For more information: Investor Relationsinvestor@anteristech.comDebbie OrmsbyAnteris Technologies Global Corp.+61 1300 550 310 | +61 7 3152 3200 Investor Relations (US)mchatterjee@bplifescience.comMalini Chatterjee, Ph.D.Blueprint Life Science Group+1 917 330 4269 Website www.anteristech.comX @AnterisTechLinkedIn https://www.linkedin.com/company/anteristech
Brainomix Joins Innovators’ Network at American Heart Association Center for Health Technology & Innovation
American Heart Association consortium advances development of evidence-based health tech solutions CHICAGO and OXFORD, England, Oct. 31, 2025 /PRNewswire/ — Brainomix, a global leader and pioneer of AI-powered imaging tools in stroke and lung fibrosis, today announced it has joined the…
BioCardia Announces University of Wisconsin Enrolls Their First Patient in Phase 3 CardiAMP HF II Cell Therapy Pivotal Trial
SUNNYVALE, Calif., Oct. 30, 2025 (GLOBE NEWSWIRE) — BioCardia, Inc. [NASDAQ: BCDA], a global leader in cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, today announced the first patient enrolled at University of Wisconsin School of Medicine and Public Health in its ongoing Phase 3 CardiAMP HF II clinical trial. “CardiAMP cell therapy has shown evidence of benefit for ischemic heart failure patients with elevated markers of heart stress, despite being on optimized medical therapy,” said Dr. Amish Raval, M.D., Professor of Medicine at UW School of Medicine and Public Health and CardiAMP HF II Trial National Co-Principal Investigator. “We look forward to offering patients the opportunity to participate in this important study and potentially contributing to the evidence that may enable this therapy to be more broadly available.” “We are pleased that the UW School of Medicine and Public Health has completed their first enrollment in our ongoing Phase 3 CardiAMP HF II clinical trial,” said Peter Altman, PhD, CEO of BioCardia. “The leadership of this prestigious cardiology center strengthens our trial. We share the vision of providing specialized medicine that translates into personalized care for improved patient outcomes. Dr. Amish Raval, who has served as our CardiAMP HF II Trial Co-National Principal Investigator since the trial’s inception, is a valued world leader in both basic science and translation of biotherapeutic interventions in cardiology. We are proud to partner with the University of Wisconsin, as well as our other CardiAMP HF II clinical sites, as they strive to provide the best in care for patients diagnosed with ischemic heart failure.” About the CardiAMP Heart Failure II Study CardiAMP HF II is a 250-patient randomized multicenter procedure placebo-controlled study of the CardiAMP autologous cell therapy as a one-time treatment for patients with ischemic heart failure with reduced ejection fraction (HFrEF) on guideline directed medical therapy having elevated NTproBNP. The study is intended to confirm the safety and efficacy results in these patients observed in the CardiAMP HF study1. The CardiAMP HF II study uses a similar three-tier composite primary outcome measure to CardiAMP HF consisting of all cause death, nonfatal major adverse cardiac events, and a validated quality of life measure. In CardiAMP HF, this composite efficacy endpoint was achieved with statistical significance in the patients with elevated NTproBNP who are the focus of the CardiAMP HF II study. Advances in this therapeutic approach for this trial include using the cell population analysis at screening to define treatment doses and improvements to the Helix system including the FDA approved Morph DNA steerable platform. About CardiAMP Autologous Cell Therapy Granted FDA Breakthrough designation, CardiAMP Cell Therapy uses a patient’s own bone marrow cells delivered to the heart in a minimally invasive, catheter-based procedure intended to increase capillary density and reduce tissue fibrosis, treating microvascular dysfunction. The CardiAMP Cell Therapy clinical development for heart failure is supported by the Maryland Stem Cell Research Fund and is reimbursed by Centers for Medicare and Medicaid Services (CMS). CAUTION – Limited by United States law to investigational use. About BioCardia BioCardia, Inc., headquartered in Sunnyvale, California, is a global leader in cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary disease. CardiAMP® autologous and CardiALLO™ allogeneic cell therapies are the Company’s biotherapeutic platforms with three clinical stage product candidates in development. These therapies are enabled by its Helix™ biotherapeutic delivery and Morph® vascular navigation product platforms. For more information visit: https://www.BioCardia.com. Forward-Looking Statements This press release contains forward-looking statements that are subject to many risks and uncertainties. Forward-looking statements include, among other things, references to our investigational product candidates, the potential benefits and mechanism of actions of the CardiAMP cell therapy, future regulatory approvals, enrollment in our clinical trials, and the safety and efficacy of our product candidates and therapies. These forward-looking statements are made as of the date of this press release, and BioCardia assumes no obligation to update the forward-looking statements. We may use terms such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or other words that convey the uncertainty of future events or outcomes to identify these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained herein, we caution you that forward-looking statements are not guarantees of future performance and that our actual results may differ materially from the forward-looking statements contained in this press release. As a result of these factors, we cannot assure you that the forward-looking statements in this press release will prove to be accurate. Additional factors that could materially affect actual results can be found in BioCardia’s Form 10-K filed with the Securities and Exchange Commission on March 26, 2025, under the caption titled “Risk Factors,” and in our subsequently filed Quarterly Reports on Form 10-Q. BioCardia expressly disclaims any intent or obligation to update these forward-looking statements, except as required by law. References: Raval A, on behalf of the CardiAMP HF Investigators. A Double-blind, Randomized Controlled Trial of an Autologous Cell Therapy in Patients with HFrEF: Principal Results from the CardiAMP-HF Trial, American College of Cardiology Scientific Sessions, March 30, 2025. CONTACT: Media Contact:
Miranda Peto, Investor Relations
Email: mpeto@BioCardia.com
Phone: 650-226-0120
Investor Contact:
David McClung, Chief Financial Officer
Email: investors@BioCardia.com
Phone: 650-226-0120
Tectonic Therapeutic Announces Positive Topline Data from Phase 1b Part B Clinical Trial for TX45 in Patients with Group 2 Pulmonary Hypertension in HFrEF
WATERTOWN, Mass., Oct. 29, 2025 (GLOBE NEWSWIRE) — Tectonic Therapeutic, Inc. (NASDAQ: TECX) (“Tectonic”), today announced positive topline results from the Phase 1b Part B acute hemodynamic clinical trial of TX45, a long-acting, Fc-relaxin fusion protein, in patients with Group 2 PH‑HFrEF. The topline data showed that a single intravenous dose of TX45 was well tolerated in this patient population and resulted in meaningful improvements in both left heart function and pulmonary hemodynamics.
Elucid Launches PlaqueIQ Image Analysis Software for the Carotid Arteries
BOSTON–(BUSINESS WIRE)–Elucid, an AI medical technology company focused on providing physicians with a more precise view of atherosclerosis to drive patient-specific therapeutic decisions, announced the launch of its PlaqueIQTM image analysis software for the quantification and classification of plaque morphology in the carotid arteries. The first and only CT-based plaque analysis […]
Biostate AI’s Indian Subsidiary Bayosthiti Partners with Narayana Health to Build World’s Largest India-Specific Cardiac AI Dataset
Landmark 12,000-patient study addresses critical gap in global healthcare: South Asian populations face heart disease at younger ages but are diagnosed with tools calibrated for Western genetics. Partnership aims to prevent heart attacks years before they occur. BENGALURU, India and…
FastWave Medical Presents First-in-Human and Pre-Clinical Data for Sola™ Coronary Laser IVL System at TCT 2025
Findings demonstrate safety, procedural success, and imaging-confirmed calcium modification using a next-generation laser intravascular lithotripsy (IVL) platform. MINNEAPOLIS, Oct. 29, 2025 /PRNewswire/ — FastWave Medical presented new first-in-human (FIH) and pre-clinical data for its…
Late-breaking iMODERN findings presented at TCT 2025 and published in the New England Journal of Medicine highlight new evidence to guide treatment choices for heart attack patients
Philips iFR technology
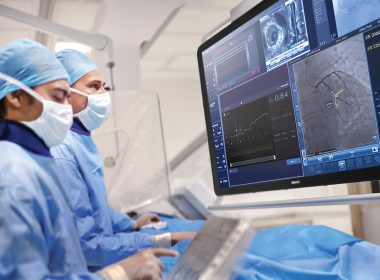
October 29, 2025 3-Year data from the largest global trial to date using Class I, Level A iFR in heart attack patients shows treating additional stenoses in the same procedure showed no significant difference in major outcomes compared with waiting for follow-upAdditional results from the ILIAS ANOCA study highlight the sustained benefits of physiology-guided assessment and tailored medical therapy for patients with angina and no obstructive coronary arteries Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced late-breaking results from the iMODERN trial (Instantaneous wave-free Ratio Guided Multivessel Revascularisation During PCI for Acute Myocardial Infarction) at the Transcatheter Cardiovascular Therapeutics (TCT) 2025 conference, the world’s leading meeting for interventional cardiology. The study compared immediate versus delayed treatment of additional narrowed arteries in heart attack patients to determine whether treating all blockages in one procedure is as safe and effective as waiting for a follow-up procedure. The study compared immediate versus deferred treatment of additional narrowed arteries in heart attack patients to determine whether treating all blockages in a single procedure is superior to waiting for a follow-up procedure – a key question, since many patients have multiple diseased arteries and the optimal timing for complete treatment remains uncertain. In today’s practice, when patients suffer a serious type of heart attack (STEMI), cardiologists urgently open the blocked artery causing the attack. But many of these patients also have disease in other arteries. Often these additional narrowed arteries are not treated right away, either due to time restraints, patient stability, resource constraints or because they stay unnoticed. They may be treated later in a separate hospital stay or not at all, leaving uncertainty about the best timing and approach. The new findings show that patients can safely have additional arteries treated immediately during the first procedure to treat the acute event, rather than during a later second intervention. By confirming the safety of extending Class I, Level A-recommended iFR to non-stable patients, the results offer physicians the opportunity to complete treatment in one session, without compromising long-term outcomes. The iMODERN trial is the largest study to date testing iFR* in the acute heart attack setting, expanding the evidence base for a tool already strongly recommended (Class I, Level A) in stable patients. The findings are published in The New England Journal of Medicine (NEJM), underscoring its global scientific significance and impact on cardiology practice. “These results address one of the longest-standing questions in interventional cardiology,” said Prof. Niels van Royen, co-principal investigator, Radboud University Medical Center, The Netherlands. “Measuring and eventually treating additional arteries can be performed during the first procedure or during a staged procedure. That means cardiologists can feel confident offering patients a complete solution in one sitting when it’s appropriate.” The iMODERN study enrolled 1,146 patients across 41 hospitals in 14 countries directly addressed this question. Patients were randomly assigned to one of two treatment strategies: either immediate physiology-guided treatment of additional narrowed arteries during the first procedure using instantaneous wave-free ratio (iFR), or staged treatment guided by cardiac Magnetic Resonance Imaging (MRI) carried out within four days to six weeks after the heart attack. The study’s main endpoint combined three outcomes: death, another heart attack, or hospitalization for heart failure over three years. After three years of follow-up, the trial found no significant difference in major outcomes – including death, repeat heart attack, or hospitalization for heart failure – between the two approaches. By confirming that both approaches are backed by solid evidence, the trial offers patients more certainty and more personalized care. “Flexibility is critical in real-world practice,” added Prof. Dr. Robin Nijveldt, co-principal investigator, at Radboud University Medical Center, in the Netherlands. “Some patients may benefit from immediate treatment, while others are better served by waiting. iMODERN is a pragmatic study that shows that an immediate intervention is not necessarily better than waiting, if patients are offered a CMR to evaluate the need for a second intervention, giving physicians the evidence they need to tailor decisions to each patient.” “These results complement current international guideline recommendations (Class I recommendation, Level A evidence) for complete revascularization in STEMI,” said Dr. Darshan Doshi, practicing interventional cardiologist and Head of Medical & Clinical at Philips Image-Guided Therapy Devices. “By integrating physiological assessment, iMODERN’s evidence demonstrates that cardiologists can follow these findings for full revascularization while also tailoring treatment to each vessel’s true ischemic relevance.” Complementary evidence from ILIAS ANOCA In related findings presented at the same conference, the ILIAS ANOCA (Inclusive Invasive Physiological Assessment in Angina Syndromes – Angina with No Obstructive Coronary Artery Disease) study further demonstrated the value of physiology-guided decision-making — this time in patients with angina and no obstructive coronary arteries (ANOCA). The ILIAS ANOCA study evaluated the impact of coronary function testing (CFT) in patients whose coronary arteries appear unobstructed on angiography but who continue to experience angina. Conducted across five cardiac centers in the Netherlands and Germany (n=153), the investigator-initiated, randomized, blinded-arm controlled trial compared standard care with CFT-guided medical therapy using Philips Doppler FloWire and FloMap systems. The study found that ad-hoc CFT followed by tailored therapy significantly improved patient-reported angina symptoms and quality of life at six months, with a mean 9.4-point gain in the Seattle Angina Questionnaire summary score (95% CI 3.9–14.9; p=0.001). There were no procedure-related complications or major adverse cardiac events. These results confirm and extend the earlier CorMiCA findings, supporting CFT as a Class I, Level A recommendation in ANOCA patients and demonstrating the potential of Philips Doppler technology to guide individualized treatment strategies safely and effectively. The 12-month results presented on October 26th demonstrate the sustained benefits of CFT-guided care. iFR and MRI technologyPhilips physiology solutions – including its instantaneous wave-free ratio (iFR) pressure wires and software – were used to guide immediate treatment decisions in the trial. Philips also provides advanced cardiac MRI technology, which guided the delayed strategy. By enabling both the invasive and non-invasive approaches evaluated in iMODERN, Philips supported the generation of robust evidence to help guide clinical practice worldwide. * iFR (instantaneous wave-free ratio) is a minimally invasive way to measure blood pressure through the coronary arteries, helping physicians decide which blockages require stenting. For further information, please contact: Joost MalthaPhilips Global External RelationsTel.: +31 6 1055816E-mail: joost.maltha@philips.com About Royal Philips Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home.Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2024 sales of EUR 18 billion and employs approximately 67,800 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachment
Philips iFR technology
Medtronic announces launch of Stedi™ Extra Support Guidewire to enhance Medtronic TAVR system performance
Stedi Extra Support Guidewire Delivers Enhanced Support for Stability, Safety, and Predictability During Valve Deployment for Aortic Stenosis Medtronic plc, a global leader in healthcare technology, today announced the launch of the Stedi™ Extra Support guidewire, designed to enhance performance of the Evolut™ transcatheter aortic valve replacement (TAVR) platform […]