– New data presented at TCT 2019 on Abbott’s TriClip device from the TRILUMINATE Feasibility Study met both the primary safety and performance endpoints – Abbott’s TriClip tricuspid valve repair system design is based on the company’s market-leading MitraClip™ technology SAN FRANCISCO, Sept. 28, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced new […]
Coronary/Structural Heart
Neovasc’s Tiara™ for Treatment of Mitral Regurgitation and Neovasc Reducer™ for Treatment of Refractory Angina Featured in Multiple Presentations at TCT 2019 Conference
VANCOUVER, Sept. 30, 2019 /PRNewswire/ – Neovasc, Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that its Tiara™ (“Tiara”) transcatheter mitral valve replacement […]
Edwards SAPIEN 3 TAVR Demonstrates Significant Health Status Improvements for Low-Risk Patients
SAN FRANCISCO, Sept. 29, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced new data demonstrating early and sustained health status advantages for severe aortic stenosis (AS) patients at low surgical risk treated with the Edwards SAPIEN 3 valve. […]
AltaValve™, 4C Medical’s Novel Transcatheter Mitral Regurgitation Device Highlighted at TCT 2019
MAPLE GROVE, Minn., Sept. 30, 2019 /PRNewswire/ — 4C Medical Technologies, Inc., the creator of a new generation of therapies for structural heart disease, announced today that its medical device therapy for mitral regurgitation (MR), AltaValve™, was featured at Transcatheter Cardiovascular Therapeutics (TCT) 2019, on Sept 25-29 in San Francisco, CA. The AltaValve is a Transcatheter […]
Resverlogix Announces Topline Results in BETonMACE Phase 3 Epigenetics Trial
BETonMACE did not meet the primary endpoint Apabetalone development to continue to be advanced by the Company based on BETonMACE results Primary results to be presented during a late-breaking science session at AHA 2019 Apabetalone demonstrated tolerability and safety Resverlogix to host investor webcast and conference call on Monday, September […]
Acasti Pharma Announces Additional Phase 3 Milestones Reached, and Remains on Track to Report Topline Results for TRILOGY 1 in December 2019 and TRILOGY 2 in January 2020
Nearly 80% of randomized patients have completed the studies Data clean-up for TRILOGY 1 is 90% completed Plan to present full data set including results for key secondary and exploratory endpoints of interest such as non-HDL-C, LDL-C, VLDL, HDL-C and HbA1c at key scientific meetings in 2020 […]
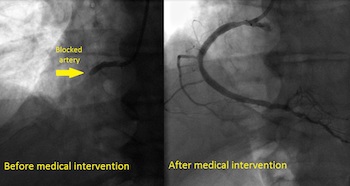
UH Portage Medical Center Achieves 6 Minute “Door-to-Balloon Time”
During a heart attack, cardiologists say “time is muscle.” The time between when you arrive at the hospital and when your blockage is opened matters. The American Heart Association recommends a “door-to-balloon time” (D2B) of no more than 90 minutes. University Hospitals Portage Medical Center in Ravenna, Ohio blew that […]
Medtronic Announces Early Feasibility Trial for Intrepid™ Transcatheter Mitral Valve Replacement System with Transfemoral Transseptal Approach
New Study Shows Momentum in Establishing a Less Invasive Approach to Treat Patients with Severe Mitral Valve Disease DUBLIN, Sept. 27, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced it has received U.S. Food and Drug Administration (FDA) approval to begin an early feasibility study (EFS) for its Intrepid™ transcatheter mitral […]
Boston Scientific Announces Positive Data from the EVOLVE Short DAPT study with the SYNERGY™ Bioabsorbable Polymer Stent
Study of abbreviated antiplatelet therapy for patients at high risk for bleeding after undergoing percutaneous coronary intervention SAN FRANCISCO and MARLBOROUGH, Mass., Sept. 26, 2019 /PRNewswire/ — Boston Scientific (NYSE: BSX) has announced primary endpoint results from the EVOLVE Short DAPT clinical trial, the first prospective study initiated in the U.S. to examine the safety […]
Resolute Onyx™ DES Meets Primary Endpoint in First-Ever Clinical Study Comparing Drug-Eluting Stents in High-Bleeding Risk (HBR) Patients with One-Month DAPT
DUBLIN and SAN FRANCISCO, Sept. 26, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) announced today late-breaking clinical data from the Onyx ONE Global Study, representing the first prospective, multi-center, randomized study evaluating clinical outcomes between two drug-eluting stents (DES) in nearly 2,000 high-bleeding risk (HBR) patients with one month of dual antiplatelet therapy […]