BOSTON, Aug. 27, 2019 (GLOBE NEWSWIRE) — LifePod® Solutions Inc., the groundbreaking, proactive-voice caregiving service, has joined the American Heart Association’s Center for Health Technology & Innovation (the Center) Innovators Network to implement a first-of-its-kind heart failure study to support CarePlan adherence among those suffering from chronic heart conditions. The Center is focused […]
Coronary/Structural Heart
Amarin Provides Overview on Growing Global Attention on REDUCE-IT™ Results and Cardiovascular Risk Management Beyond Cholesterol Management
Multiple Papers and Presentations Highlight REDUCE-IT and Vascepa® Potential Mechanisms of Action of Vascepa Becoming Better Understood American Heart Association References REDUCE-IT in Scientific Advisory BEDMINSTER, N.J. and DUBLIN, Ireland, Aug. 27, 2019 (GLOBE NEWSWIRE) — Amarin Corporation plc (NASDAQ:AMRN), a pharmaceutical company focused on improving cardiovascular health, today provided […]
Miracor Medical Granted FDA Breakthrough Device Designation for the PiCSO Impulse System
AWANS, Belgium–(BUSINESS WIRE)–Miracor Medical SA (Miracor Medical) has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for its PiCSO® Impulse System for treatment of STEMI patients. The FDA Breakthrough Device designation is intended to speed time to market for treatments of life-threatening or irreversibly debilitating diseases […]
MyoKardia Begins Dosing in Phase 1 Clinical Study of MYK-224 for Hypertrophic Cardiomyopathy
SOUTH SAN FRANCISCO, Calif., Aug. 21, 2019 (GLOBE NEWSWIRE) — MyoKardia, Inc. (Nasdaq: MYOK) today announced that it has dosed the first subjects in a Phase 1 clinical study of MYK-224, a small molecule candidate being developed for the treatment of hypertrophic cardiomyopathy (HCM). HCM is a progressive disease in which […]
FARXIGA Met Primary Endpoint in Landmark Phase III DAPA-HF Trial for the Treatment of Patients With Heart Failure
DAPA-HF is the first heart failure outcomes trial with an SGLT2 inhibitor in patients with and without type 2 diabetes FARXIGA significantly reduced the risk of cardiovascular death or worsening of heart failure when added to standard of care WILMINGTON, Del.–(BUSINESS WIRE)–AstraZeneca today announced positive results from the landmark Phase […]
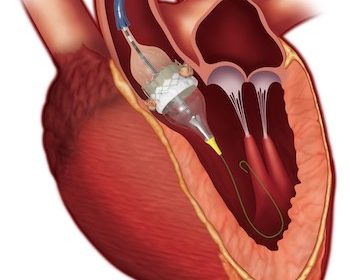
Medtronic Evolut TAVR System Receives Expanded Indication to Treat Symptomatic Severe Aortic Stenosis Patients at Low Risk for Surgical Mortality
DUBLIN, Aug. 16, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) approval of the Evolut™ Transcatheter Aortic Valve Replacement (TAVR) system for patients with symptomatic severe native aortic stenosis who are at a low risk of surgical mortality. The low-risk patient population is […]
Edwards SAPIEN 3 TAVR Receives FDA Approval For Low-Risk Patients
IRVINE, Calif., Aug. 16, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced U.S. Food and Drug Administration (FDA) approval to expand use of the Edwards SAPIEN 3 and SAPIEN 3 Ultra transcatheter heart valve systems to the treatment […]
MyoKardia Launches 2nd Annual MyoSeeds™ Research Grants Program to Advance Independent Research in Heart Disease
Pre-Application Letters of Intent are Due September 13 for the 2019-2020 Funding Cycle SOUTH SAN FRANCISCO, Calif., Aug. 15, 2019 (GLOBE NEWSWIRE) — MyoKardia, Inc. today announced the launch of the 2nd Annual MyoSeeds™ Research Grants Program, an initiative to support original, independent research in the biology and underlying mechanisms of cardiomyopathies […]
preCARDIA Enrolls First Patient in Early Feasibility Study
Company initiates the VENUS-HF study in patients with Acute Decompensated Heart Failure (ADHF) ST. PAUL, Minn., Aug. 15, 2019 /PRNewswire/ — preCARDIA, Inc., a medical device company developing innovative technologies for the treatment of acute decompensated heart failure (ADHF), has enrolled the first patient in the VENUS-HF Early Feasibility Study at Tufts Medical […]
V-Wave’s Interatrial Shunt Receives FDA Breakthrough Device Designation for Heart Failure
CAESAREA, Israel, Aug. 15, 2019 /PRNewswire/ — V-Wave Ltd., a privately held medical device company developing novel implantable interatrial shunt devices, today announced that the U.S. Food and Drug Administration (FDA) has just granted the company a Breakthrough Device Designation for its interatrial shunt for Heart Failure (HF). V-Wave’s minimally invasive, implanted interatrial […]