– Funding supports preclinical development through IND submission for its BAG3 gene therapy, the first gene replacement product for genetic forms of dilated cardiomyopathy – Company announces leadership team and board of directors with leading scientists, physicians and experienced venture investors in cardiovascular and gene therapy fields August 14, 2019 10:11 AM […]
Coronary/Structural Heart
Ancora Heart Enrolls First Patient in European Multi-Center Study of First-of-Its-Kind Investigational Heart Failure Therapy
Study will evaluate a percutaneous therapy designed to improve left ventricular function for patients with systolic heart failure SANTA CLARA, Calif.–(BUSINESS WIRE)–Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced the first patient was enrolled in the CorCinch EU study, a European multi-center clinical […]
BioCardia Announces Issuance of European Patent for Breakthrough Diagnostic Assay for Patient Selection Prior to Cardiac Cell Therapy Delivery
SAN CARLOS, Calif., Aug. 08, 2019 (GLOBE NEWSWIRE) — BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced that the European Patent Office has issued the Company Patent No: 3063172 for “Methods of Measuring Potential for Therapeutic Potency and Defining Dosages for Autologous […]
The Children’s Heart Foundation and Cardiac Networks United Partner to Advance Congenital Heart Research
NORTHBROOK, Ill., Aug. 5, 2019 /PRNewswire/ — The Children’s Heart Foundation, the nation’s leading organization dedicated to funding congenital heart research, has announced a new partnership to advance the diagnosis, treatment, and prevention of congenital heart defects. The foundation has committed $1.5 million in funding over the next five years to the University of Michigan Congenital Heart […]
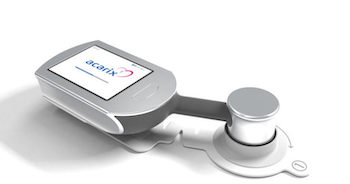
CADScor®System – Significantly more effective than current practice
Malmö August 5, 2019 CADScor®System – Significantly more effective than current practice Acarix AB (publ) (ACARIX: FN Stockholm) today announced the publication of a meta-analysis including 2245 patients showing Acarix’s leading CADScor®System is more than three times as effective as current practice, implying clinical and economic advantages. “Strong clinical evidence […]
B. Braun Interventional Systems Receives Breakthrough Device Designation Status from the FDA for SeQuent Please ReX Drug Coated PTCA Balloon Catheter
BETHLEHEM, Pa., Aug. 01, 2019 (GLOBE NEWSWIRE) — B. Braun Interventional Systems Inc. (BIS) announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the SeQuent Please ReX drug-coated PTCA balloon catheter for the treatment of coronary in-stent restenosis (ISR). SeQuent Please ReX is […]
Endotronix Announces FDA Approval to Begin Landmark PROACTIVE-HF Pivotal Trial for Cordella™ PA Pressure Sensor System
LISLE, Ill., Aug. 1, 2019 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), today announced it has received conditional Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to begin the multi-center PROACTIVE-HF trial of the Cordella™ Pulmonary […]
FRESH 3D Printing Used to Rebuild Functional Components of Human Heart
ACTON, Mass., Aug. 1, 2019 /PRNewswire/ — Scientists have taken a major step closer to being able to 3D bioprint functional organs, after researchers devised a method of rebuilding components of the human heart, according to a study published in the August 2nd edition of Science. The team of Carnegie Mellon University researchers developed an advanced version […]
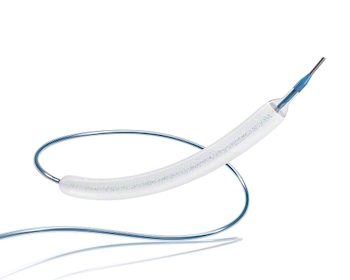
Prix Galien Selects BIOTRONIK’s PK Papyrus Covered Coronary Stent as Award Finalist
LAKE OSWEGO, Ore., July 31, 2019 /PRNewswire/ — BIOTRONIK today announced the company’s PK Papyrus® covered coronary stent system1 has been named a finalist in the 2019 Prix Galien USA Awards Ceremony for Best Medical Technology. PK Papyrus is used in emergency situations to create a physical barrier to seal life-threatening perforated coronary arteries without the need for invasive surgery. In September […]
Reflow Medical Enrolls First Patients in DEEPER OUS Spur Stent Trial
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc. announces that the first patients have been enrolled in its DEEPER OUS clinical trial using the Temporary Spur Stent System. DEEPER OUS is a 100-patient prospective, non-randomized, multicenter trial designed to assess the safety and efficacy of the Temporary Spur Stent System compared to […]