WHIPPANY, N.J., July 15, 2019 /PRNewswire/ — Bayer announced today the U.S. Food and Drug Administration (FDA) has approved Gadavist® (gadobutrol) injection for use in cardiac magnetic resonance (MR) imaging to assess myocardial perfusion (stress, rest) and late gadolinium enhancement in adult patients with known or suspected coronary artery disease (CAD). Gadavist is […]
Coronary/Structural Heart
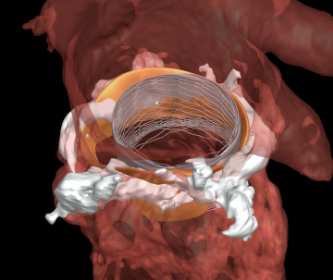
Abbott Receives U.S. Approval of Next-Generation MitraClip®, Bringing New Enhancements to Abbott’s Leading MitraClip Platform
ABBOTT PARK, Ill., July 15, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced the company has received U.S. Food and Drug Administration (FDA) approval for the most advanced MitraClip™ heart valve repair device to treat mitral regurgitation. The latest approval for the fourth-generation MitraClip device, MitraClip G4, puts new enhancements into the hands of […]
US Government to Transform Kidney Care
12 July, 2019 – Minnesota, United States and Melbourne, Australia – Osprey Medical (ASX: OSP) today announces its response to the US President signing an Executive Order aimed at transforming kidney care for more than 37 million Americans with some form of kidney disease. The initiative seeks to prevent kidney […]
MeRes100 BRS – the World’s First Thin Strut Sirolimus-Eluting Bioresorbable Vascular Scaffold – Shows Consistent Long-term Positive Safety With Consistent Low MACE rates and Zero Scaffold Stent Thrombosis for Patients With Coronary Artery Disease
LONDON, July 11, 2019 /PRNewswire/ — Meril’s recently CE-approved MeRes100, used for the treatment of de-novocoronary artery lesions, has demonstrated zero scaffold thrombosis and very low Major Adverse Cardiac Event (MACE) rate of 1.87% at three years as shown in MeRes-1 and 1.61% in MeRes-1 Extend at two years. “First generation bioresorbable scaffolds have not […]
LivaNova Celebrates 25 Years of Vagus Nerve Stimulation Therapy
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, is celebrating the 25th anniversary of its Vagus Nerve Stimulation Therapy® (VNS Therapy) System. This clinically proven, safe and effective therapy is used for the treatment of Drug-Resistant Epilepsy (DRE), Treatment-Resistant Depression (TRD) and for the delivery of Autonomic Regulation Therapy for […]
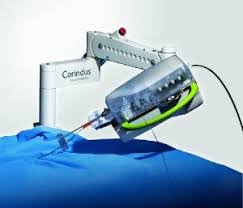
Corindus Announces First Commercial Installation of CorPath® GRX System in South America
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. (“Corindus” or the “Company”) (NYSE American: CVRS), a leading developer of precision vascular robotics, announced today that Albert Einstein Jewish Hospital (Hospital Israelita Albert Einstein) in São Paulo, Brazil has become the first hospital in the Southern Hemisphere to implement Corindus’ CorPath GRX System. Ranked as the […]
Resverlogix’s BETonMACE Phase 3 Epigenetics Trial Completes Final Safety Visits
CALGARY, Alberta, July 08, 2019 (GLOBE NEWSWIRE) — Resverlogix Corp. (“Resverlogix” or the “Company”) (TSX:RVX) is pleased to announce today that all patients active in BETonMACE, the Company’s event-based, phase 3 registration trial, recently completed their final, follow-up safety visits, marking another important step towards trial completion. Given the remaining steps […]
Quantum Genomics Selected to Enter the European Rising Tech Label
PARIS and NEW YORK, July 03, 2019 (GLOBE NEWSWIRE) — Quantum Genomics (Euronext Growth – FR0011648971 – ALQGC), a biopharmaceutical company specializing in the development of a new drug class that directly targets the brain to treat resistant hypertension and heart failure, has been selected to enter the European Rising Tech […]
FEops HEARTguideTM Wins CSI Highlight of the Year Award
GENT, Belgium–(BUSINESS WIRE)–FEops, a leader in personalized predictive planning for structural heart interventions, is proud to announce that FEops HEARTguideTM was the winning entry at CSI Frankfurt 2019 voted on by physician attendees for the Highlight of the year award in the category interventional imaging. This award celebrates promising technologies that will have the […]
Eko Unveils Algorithm to Assist in the Detection of Aortic Stenosis, a Valvular Heart Disease Affecting Over 3 Million People in the U.S.
Berkeley, June 28, 2019 (GLOBE NEWSWIRE) — Eko, a Berkeley-based digital health company applying artificial intelligence and machine learning in the fight against heart disease, unveiled an investigational Aortic Stenosis (AS) detection algorithm at the American Society of Echocardiography (ASE) 2019 Scientific Sessions aimed to assist healthcare providers in the early detection of […]