WASHINGTON, June 17, 2019 /PRNewswire/ — Ahead of a final decision from the Centers for Medicare & Medicaid Services (CMS) that may continue to limit Medicare coverage for a heart valve disease treatment called transcatheter aortic valve replacement (TAVR), a new survey shows that Americans support expansion of access to the procedure for […]
Coronary/Structural Heart
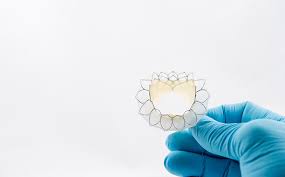
Tiara™ Featured in Presentation at 11th Annual TVT 2019
VANCOUVER, June 17, 2019 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that its Tiara™ (“Tiara”) transcatheter mitral valve replacement […]
CHF Solutions Sponsored AATS Session on Post-Cardiac Surgery, Highlighting Benefits of Using the Aquadex FlexFlow® System Following Cardiac Surgery
EDEN PRAIRIE, Minn., June 13, 2019 (GLOBE NEWSWIRE) — CHF Solutions (Nasdaq: CHFS), today announced highlights from the sponsored session from the American Association for Thoracic SurgeryAnnual Meeting moderated by Daniel Beckles, M.D., Ph.D. of United Health Services Hospitals in Binghamton, N.Y. The full session can be found on the company’s website at www.chf-solutions.com. The panel, titled “Personalized Medicine: […]
Surgical Theater’s Precision VR® featured during Late-Breaking Science Sessions at the Transcatheter Valve Therapies 2019 Structural Heart Summit
CHICAGO, June 13, 2019 /PRNewswire/ — Leveraging its rich history of providing surgeons and their patients dynamic visualization experiences in the clinic and throughout the treatment continuum, Surgical Theater is proud that the Precision VR® platform is being featured at the Transcatheter Valve Therapies (TVT) Structural Heart Summit’s Late-Breaking Science Sessions in Chicago, IL. […]
Materialise Receives FDA Clearance for Cardiovascular Planning Software Suite
CHICAGO–(BUSINESS WIRE)–TVT2019 – Structural Heart Summit — Materialise (NASDAQ:MTLS), a global 3D printing software and solutions company, has received FDA clearance for its Mimics Enlight cardiovascular planning software suite. The first release will support clinicians planning complex transcatheter mitral valve replacement (TMVR) procedures. Mimics Enlight is based on the strengths of […]
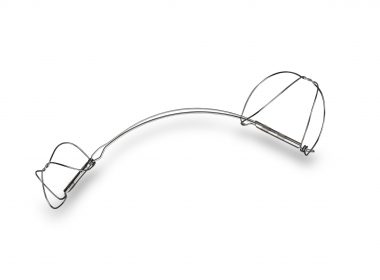
New Data Shows The Carillon Mitral Contour System® Is Associated With Improvement In Regurgitant Volume In Patients With Increased Mitral Valve Tenting
CHICAGO, June 13, 2019 /PRNewswire/ — Cardiac Dimensions, a leader in the development of minimally invasive treatments for functional mitral regurgitation (FMR) in patients with heart failure, presented data at the TVT2019 Structural Heart Summit showing increased mitral valve tenting is associated with an improvement in regurgitant volume with the Carillon Mitral Contour […]
Terumo Enhances Coronary Interventional Product Portfolio By Securing Exclusive Global Rights to Orchestra BioMed’s Virtue® Sirolimus-Eluting Balloon (SEB) through Global Strategic Partnership
TOKYO, June 13, 2019 /PRNewswire/ — Terumo Corporation (TSE: 4543), a leading global medical device manufacturer, today announced that it has secured exclusive global development and commercialization rights to Virtue® Sirolimus-Eluting Balloon (SEB) for percutaneous coronary interventions by entering into a strategic partnership with Orchestra BioMed, Inc., a biomedical innovation company providing high-impact solutions […]
BioCardia Announces Issuance of Chinese Patent for Image-Guided Biotherapeutic Delivery
SAN CARLOS, Calif., June 12, 2019 (GLOBE NEWSWIRE) — BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced that the China National Intellectual Property Administration has recently issued the Company Patent No: ZL 2014 8 00125575 for “Target Site Selection, Entry and Update […]
Transseptal Solutions Announces First Clinical Use of TSP Crosser™ in the United States
NETANYA, Israel, June 12, 2019 /PRNewswire/ — Transseptal Solutions Ltd., developer of an innovative Transseptal Access System with a novel approach of transseptal puncture and left atrial navigation, announced today the completion of the first TSP Crosser™ transseptal puncture procedure in the US. The procedure was successfully performed by Dr. Latib, the New Medical Director of […]
CorMatrix® Cardiovascular, Inc. receives FDA 510(k) clearance to market the Cor™ PATCH epicardial patch for tissue support and repair in adult patients
ATLANTA, June 11, 2019 /PRNewswire/ — CorMatrix® Cardiovascular, Inc. www.cormatrix.com, a leading developer of regenerative cardiovascular medical devices, today announced FDA 510(k) clearance for the Cor™ PATCH. The Cor™ PATCH is indicated for epicardial tissue support and repair in adult patients. The Cor™ PATCH epicardial patch is the first epicardial cardiovascular medical device composed of first next […]