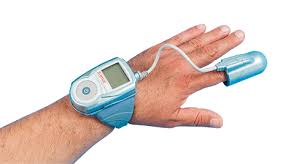
CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL® Medical Corporation, an Asahi Kasei Group company that manufactures medical devices and related software solutions, announced the launch of and first patient prescription for the ZOLL µCor™ (pronounced “Micro Core”) Heart Failure and Arrhythmia Management System (HFAMS), designed to help clinicians improve outcomes and reduce hospitalizations for […]
Coronary/Structural Heart
Precision Wellness Validates Performance of Predictive Risk Models to Power Precision Health
PALO ALTO, Calif.–(BUSINESS WIRE)–Precision Wellness, Inc. a Silicon Valley-based company at the intersection of next-generation AI, big data analytics and the latest medical science, today announced the validation of its cardiometabolic disease (CMD) predictive risk engine. The study was performed as part of a sponsored research with Stanford University to evaluate 416,936 […]
Itamar Medical’s WatchPAT™ One, the First and Only Fully Disposable Home Sleep Apnea Test Receives FDA 510(k) Clearance
New system expands WatchPAT access to additional sleep centers by reducing infrastructure needs and eliminating capital requirements; also reduces infection risk for in-patient sleep apnea testing CAESAREA, Israel, June 06, 2019 (GLOBE NEWSWIRE) — Itamar Medical Ltd. (Nasdaq:ITMR) (TASE:ITMR), a company that develops, manufactures and markets non-invasive diagnostic medical devices […]
Quantum Genomics Enrolls First Patient in QUORUM Phase IIb Study of Firibastat in Heart Failure Patients
PARIS and NEW YORK, June 06, 2019 (GLOBE NEWSWIRE) — Quantum Genomics (Euronext Growth – FR0011648971 – ALQGC), a biopharmaceutical company specializing in the development of a new drug class that directly targets the brain to treat resistant hypertension and heart failure, announces the enrollment of the first patient in its QUORUM […]
Getinge Opens New Acute Care Section in the Experience Center in Germany
GETINGE, Sweden, June 5, 2019 /PRNewswire/ — Getinge has opened up a brand new acute care section focused on Cardiovascular Procedures in one of its Experience Centers located in Rastatt, Germany. Here, customers have the possibility to learn more about the solutions that Getinge provides to not only aid in the Coronary Artery Bypass Surgery […]
Itamar Medical and BioTel Heart, a Division of BioTelemetry, Expand Collaboration to Offer Itamar’s Total Sleep Solution™ to BioTel Heart Cardiology Customers
CAESAREA, Israel, June 04, 2019 (GLOBE NEWSWIRE) — Itamar Medical Ltd. (NASDAQ and TASE:ITMR), a company that develops, manufacturers and markets non-invasive diagnostic devices for sleep apnea with a focus on the cardiology market, and BioTel Heart, a division of BioTelemetry, Inc. (NASDAQ:BEAT), the leading remote medical technology company focused […]
GORE® CARDIOFORM ASD Occluder Receives FDA Approval for the Treatment of Atrial Septal Defects
FLAGSTAFF, Ariz., June 4, 2019 /PRNewswire/ — W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket approval (PMA) of the GORE® CARDIOFORM ASD Occluder for the percutaneous closure of ostium secundum atrial septal defects (ASDs). The FDA approval was supported by data collected from the pivotal stage of the Gore […]
Ancora Heart Enrolls First Patient in U.S. Early Feasibility Study of First-of-Its-Kind Investigational Heart Failure Therapy
SANTA CLARA, Calif.–(BUSINESS WIRE)–Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced the first patient was enrolled in a U.S. early feasibility study evaluating the AccuCinch® Ventricular Repair System as a treatment for patients with reduced ejection fraction systolic heart failure (HFrEF). The first patient […]
MyoKardia Presents Results from Phase 1a Clinical Trial of MYK-491 at the European Society of Cardiology Heart Failure Congress in Athens, Greece
First-in-Human Data for MYK-491 in Healthy Volunteers Demonstrated Well-Tolerated Increases in Cardiac Contractility Topline Results from Phase 2a Clinical Trial of MYK-491 in Patients with Stable Heart Failure with Reduced Ejection Fraction Anticipated in Second Half 2019 SOUTH SAN FRANCISCO, Calif. and ATHENS, Greece, May 28, 2019 (GLOBE NEWSWIRE) — […]
Sensible Medical Innovations Licenses ReDS™ Technology to Bayer
NETANYA, Israel, May 28, 2019 /PRNewswire/ — Sensible Medical Innovations and Bayer successfully signed an agreement making Bayer, Sensible’s largest customer in Europe. Bayer will use Sensible ReDS™ technology as an exploratory device-derived biomarker to monitor lung congestion in a clinical trial (ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03901729). The ReDS System is a wearable vest which quantifies the amount of […]