PARIS, May 22, 2019 /PRNewswire/ — Pi-Cardia Ltd. announced today that it successfully completed its First-in-Human study demonstrating substantial improvement in valve function following treatment with its Leaflex™ Performer catheter. The Leaflex™ Performer is a transfemoral catheter that uses two unique mechanical structures for scoring valve calcification at multiple locations, restoring leaflets […]
Coronary/Structural Heart
WomenHeart Celebrates 20 Years of Service and Honors Women’s Heart Health Advocates at Annual Wenger Awards
WASHINGTON–(BUSINESS WIRE)–WomenHeart: The National Coalition for Women with Heart Disease recognized contributions to women’s cardiovascular health at its 2019 Wenger Awards. The organization also celebrated 20 years of providing support, education and advocacy for the 48 million women living with or at risk of heart disease. “It’s been an incredible […]
First patient treated in DIAMOND study to evaluate if Veltassa® (patiromer) improves outcomes by enabling long-term use of essential RAASi therapy
ZURICH–(BUSINESS WIRE)–Regulatory News: Vifor Pharma today announced that treatment of the first patient in their global phase-IIIb DIAMOND study has begun. The study will evaluate the potential of Veltassa® to improve outcomes by enabling heart failure (HF) patients, with or without chronic kidney disease (CKD), to be treated with renin-angiotensin-aldosterone system […]
Late-Breaking Data at EuroPCR: BIOTRONIK’s Orsiro Demonstrates Unique Benefits in Small Vessels
PARIS and BUELACH, Switzerland, May 21, 2019 /PRNewswire/ — Focusing on patients with small vessels, three-year outcomes of the BIO-RESORT randomized controlled trial (RCT) showed a significantly lower target lesion revascularization (TLR) rate and thus better efficacy of the Orsiro® drug-eluting stent1 (DES) in comparison to the Resolute Integrity DES.2 Prof. Clemens von Birgelen, Thoraxcentrum Twente, MST, Enschede, the […]
World-wide Study Finds Linoleic Acid Benefits the Heart
ALBANY, Ga., May 21, 2019 /PRNewswire/ — A large-scale study recently published in the American Heart Association’s journal, Circulation, showed that higher levels of linoleic acid in the body are associated with a lower risk of major cardiovascular events. Specifically, linoleic acid, the main omega-6 fat found in peanuts, was associated with a 22-percent […]
ARTEREZ is Targeting the Multi-Factorial ‘Root-Causes’ Of Cardiovascular Disease While Exposing the Cholesterol Hypothesis Myth. How Did it all Get Started?
METAMORA, Mich., May 21, 2019 /PRNewswire/ — In 1908, a Russian scientist (Alexander Ignatowski) thought that eating too much protein would speed up the aging process in humans. To test this hypothesis, he fed rabbits meat, milk and eggs and found ‘fatty streaks’ accumulated in the arteries. In 1913, Nikolai Anichkov, repeated the […]
National Cardiogenic Shock Initiative (NCSI) with Impella Best Practices Demonstrates 72% Survival with 98% Native Heart Recovery
LAS VEGAS–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces that new data from the National Cardiogenic Shock Initiative Study (NCSI) on 171 consecutive AMI cardiogenic shock (AMICS) patients from 35 sites demonstrates 72% survival with 98% native heart recovery at discharge. The patients were treated with the NCSI protocol, which includes placing the Impella heart pump before […]
TRILUMINATE Study Shows Treatment with Abbott’s First-of-its-Kind Minimally Invasive Device Reduces Tricuspid Heart Valve Leaks
PARIS, May 21, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced positive late-breaking data from its TRILUMINATE study of the company’s minimally invasive tricuspid valve repair system. Results at 30 days demonstrated that the investigational device is associated with a reduction of tricuspid regurgitation (TR) symptoms – caused by a leaky tricuspid heart valve […]
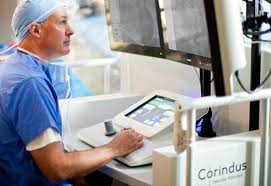
Corindus to Showcase CorPath® GRX Robotic System at EuroPCR 2019
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. (“Corindus” or the “Company”) (NYSE American: CVRS), a leading developer of precision vascular robotics, announced today that it will showcase advanced robotic capabilities of the CorPath GRX System at EuroPCR 2019 in Paris, France from May 21-24, 2019. Corindus will use the annual meeting at EuroPCR […]
Impella SmartAssist Platform Launches at SCAI, Designed to Further Improve Patient Outcomes
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces today that the Impella CP with SmartAssist, which is designed to improve patient outcomes with advanced algorithms and simplified patient management, will be commercially available beginning at the 2019 Society for Cardiovascular Angiography & Interventions (SCAI) Scientific Sessions through a controlled launch process at select sites. The […]