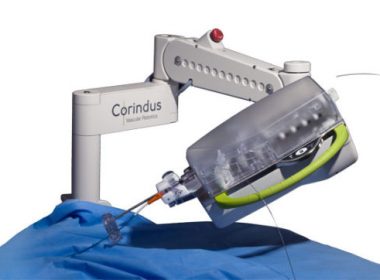
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. (“Corindus” or the “Company”) (NYSE American: CVRS), a leading developer of precision vascular robotics, announced today that Chesapeake Regional Healthcare in Chesapeake, VA has equipped both of its catheterization labs at Chesapeake Regional Medical Center with CorPath GRX Vascular Robotic Systems, making it the first hospital […]
Coronary/Structural Heart
FDA approves new treatments for heart disease caused by a serious rare disease, transthyretin‑mediated amyloidosis
SILVER SPRING, Md., May 6, 2019 /PRNewswire/ — On May 3, the U.S. Food and Drug Administration approved Vyndaqel (tafamidis meglumine) and Vyndamax (tafamidis) capsules for the treatment of the heart disease (cardiomyopathy) caused by transthyretin‑mediated amyloidosis (ATTR-CM) in adults. These are the first FDA-approved treatments for ATTR-CM. Vyndaqel and Vyndamax have the […]
Neovasc Announces Treatment of 1,000th Refractory Angina Patient with Neovasc Reducer™
VANCOUVER, May 6, 2019 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN)(TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, announced today that 1,000 patients diagnosed with refractory angina have been […]
FDA approves new treatments for heart disease caused by a serious rare disease, transthyretin‑mediated amyloidosis
SILVER SPRING, Md., May 6, 2019 /PRNewswire/ — On May 3, the U.S. Food and Drug Administration approved Vyndaqel (tafamidis meglumine) and Vyndamax (tafamidis) capsules for the treatment of the heart disease (cardiomyopathy) caused by transthyretin‑mediated amyloidosis (ATTR-CM) in adults. These are the first FDA-approved treatments for ATTR-CM. Vyndaqel and Vyndamax have the […]
New Data Emphasizing LivaNova Perceval Valve Durability to be Presented at the American Association for Thoracic Surgery Meeting
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, today announced that new clinical data for its sutureless surgical aortic valve, Perceval®, will be unveiled at this year’s American Association for Thoracic Surgery (AATS) meeting. On May 4, Prof. Bart Meuris from Leuven University Hospital (BE) will present the data […]
Concept Medical Inc. Granted ‘Breakthrough Device Designation’ From FDA for Its MagicTouch Sirolimus Coated Balloon
TAMPA, Florida, May 2, 2019 /PRNewswire/ — Concept Medical Inc. (CMI) has been granted “Breakthrough Device Designation” from the U.S. Food and Drug Administration (FDA) for MagicTouch, its Sirolimus drug-coated balloon (DCB) catheter, for the treatment of coronary in-stent restenosis (ISR). In-stent restenosis (ISR) is the gradual re-narrowing of a stented coronary artery lesion, due to […]
FDA Approves Initiation of STEMI DTU Pivotal Randomized Controlled Trial
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces that, on April 26, the FDA approved initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial. The prospective, multi-center, two-arm trial plans to enroll 668 patients undergoing treatment for a STEMI heart attack. Half the patients will be randomized to receive […]
Clinical Trial Results from MyoKardia’s Phase 2 PIONEER-HCM Study of Mavacamten Published in the Annals of Internal Medicine
SOUTH SAN FRANCISCO, Calif., April 30, 2019 (GLOBE NEWSWIRE) — MyoKardia, Inc. (Nasdaq: MYOK), a clinical-stage biopharmaceutical company pioneering a precision medicine approach for the treatment of serious cardiovascular diseases, today announced the publication of results from the company’s Phase 2 PIONEER-HCM study of mavacamten in an upcoming issue of […]
OPSENS SIGNS LANDMARK SUPPLIER AGREEMENT – EXPANDS OPTICAL SENSING TECHNOLOGY IMPACT WITHIN CARDIOLOGY SEGMENT
Quebec City, Quebec, April 30, 2019 – Opsens inc. (“Opsens” or the “Company”) (TSX:OPS) (OTCQX:OPSSF) is pleased to announce it has entered into a supply agreement as part of its long-term collaboration with Abiomed, Inc. (“Abiomed”) for the Impella CP® heart pump. Abiomed has awarded Opsens a five-year agreement to supply […]
New Study Finds Abbott Blood Test Can Help Predict Future Cardiac Events in Adults with No Known Heart Disease
ABBOTT PARK, Ill., April 29, 2019 /PRNewswire/ — Abbott (NYSE: ABT) announced today that a new study published in Circulation found its High Sensitive Troponin-I blood test could predict the chance of developing a cardiac event years before it occurs in people with no symptoms when added to current heart disease assessments.1 Using data from the Atherosclerosis […]