CALGARY, Alberta, March 27, 2019 (GLOBE NEWSWIRE) — Resverlogix Corp. (“Resverlogix” or the “Company”) (TSX:RVX) today announces the independent Data and Safety Monitoring Board (DSMB) has completed a ninth planned safety review for the Company’s Phase 3, BETonMACE trial for high-risk cardiovascular disease patients and recommended the study continue without modification. The […]
Coronary/Structural Heart
Newly Published Article Demonstrates Ultrafiltration with CHF Solutions’ Aquadex FlexFlow® System Reduces Hospital Costs by Lowering 90-Day Re-admissions and Durations of Stay in Heart Failure Patients
EDEN PRAIRIE, Minn., March 26, 2019 (GLOBE NEWSWIRE) — CHF Solutions (Nasdaq: CHFS), today announced the publication of “Ultrafiltration vs. Diuretics for Treatment of Fluid Overload in Patients with Heart Failure: A Hospital Cost Analysis” in the Journal of Medical Economics showing that the use of ultrafiltration therapy with the company’s Aquadex […]
Neovasc Wins German Court Appeal; Announces German Court’s Decision to Dismiss CardiAQ’s Claim to Co-inventorship of a European Patent for Tiara™
VANCOUVER, March 21, 2019 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN)(TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that the Higher Regional Court in Munich, Germany, on appeal […]
Zenosense, Inc.: Update on MIDS Medical Test Strip Development
VALENCIA, SPAIN, March 20, 2019 (GLOBE NEWSWIRE) — via NEWMEDIAWIRE — Zenosense, Inc. (OTCQB: ZENO, “Zenosense”, the “Company”), a healthcare technology company focused on the development and commercialization of the MIDS Cardiac™ hand-held technology for the early detection of heart attack at the Point of Care, is pleased to announce an […]
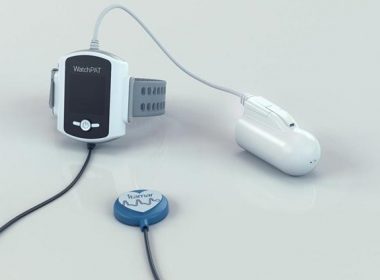
Itamar Medical Advances Simple, Accurate and Reliable Home Sleep Apnea Testing with Launch of WatchPAT™ 300
CAESAREA, Israel, March 20, 2019 (GLOBE NEWSWIRE) — Itamar Medical Ltd. (Nasdaq & TASE: ITMR), a company that develops, manufactures and markets non-invasive diagnostic medical devices for sleep apnea with a focus on the cardiology market, today announced the launch of WatchPAT 300, the next generation WatchPAT system for home sleep […]
Quantum Genomics Announces Positive Results from New Preclinical Studies on Firibastat
PARIS and NEW YORK, March 20, 2019 (GLOBE NEWSWIRE) — Quantum Genomics (Euronext Growth – FR0011648971 – ALQGC), a biopharmaceutical company announces positive results from preclinical reproductive toxicity studies conducted in rats and rabbits with firibastat, a first-in-class cerebral aminopeptidase A inhibitor (BAPAI, Brain Aminopeptidase A Inhibitor). The results of these preclinical […]
Hoag Clinical Trial Contributes to ‘Eye-Popping’ National Heart Surgery Data
NEWPORT BEACH, Calif., March 18, 2019 /PRNewswire/ — The Jeffrey M. Carlton Heart & Vascular Institute at Hoag, the largest volume cardiovascular program in Orange County, was the first hospital to bring transcatheter aortic valve replacement (TAVR) to the community. Today, the American College of Cardiology (ACC) released data from two trials showing […]
Impella RP Post-Approval Study Data Presented at ACC 2019
March 18, 2019 07:00 AM Eastern Daylight Time NEW ORLEANS, La.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD), a leading provider of breakthrough heart support technologies and the maker of the Impella RP heart pump, announces that survival data from the 18 month post-approval study of 42 Impella RP patients was presented at the American College of Cardiology’s […]
FDA approves new indication for valve repair device to treat certain heart failure patients with mitral regurgitation
SILVER SPRING, Md., March 14, 2019 /PRNewswire/ — The U.S. Food and Drug Administration today approved a new indication for a heart valve repair device that is intended to reduce moderate-to-severe or severe mitral regurgitation, a leakage of blood backward through the mitral valve into the heart’s left atrium that can cause […]
ABBOTT RECEIVES FDA APPROVAL FOR EXPANDED INDICATION FOR MITRACLIP™ DEVICE
ABBOTT PARK, Ill., March 14, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced it received approval from the U.S. Food and Drug Administration (FDA) for a new, expanded indication to its leading MitraClip™ device used to repair a leaky mitral valve without open-heart surgery. Supported by the results of the landmark COAPT™ Trial, […]