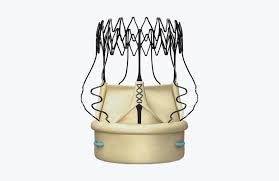
BROOKLYN PARK, Minn., Feb. 25, 2019 /PRNewswire/ — 4C Medical Technologies, Inc. (4C Medical), a developer of minimally invasive technologies for structural heart disease, today announced that its AltaValve™, a transcatheter mitral valve replacement (TMVR) technology, will be highlighted in the following presentations at the Cardiovascular Research Technologies (CRT) meeting held March 2-5, 2019 in Washington, […]
Coronary/Structural Heart
LivaNova’s Perceval Sutureless Aortic Heart Valve Receives National Reimbursement in Japan to Treat Patients with Aortic Valve Disease
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology company, today announced that Japan’s Ministry of Health, Labour and Welfare granted national reimbursement for the Company’s Perceval® sutureless aortic heart valve to treat aortic valve disease. By adding Perceval into Japan’s health insurance system, physicians and patients have greater access to this […]
Acasti Pharma Recognized in TSX Venture Exchange’s 2019 Venture 50
LAVAL, Quebec, Feb. 21, 2019 (GLOBE NEWSWIRE) — Acasti Pharma Inc. (“Acasti” or the “Company”) (NASDAQ: ACST – TSX-V: ACST), a biopharmaceutical innovator focused on the research, development and commercialization of its prescription drug candidate CaPre® (omega-3 phospholipid) for the treatment of severe hypertriglyceridemia, announces it has been recognized by […]
Neovasc Celebrates 5-Year Anniversary of Tiara Patient as the Longest Surviving Transcatheter Mitral Valve Replacement in the World
VANCOUVER, Feb. 21, 2019 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN) (TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that a patient implanted with a Tiara™ transcatheter […]
Rebirth of a Heart Valve: Franciscan Health Surgeon First in World to Use Device
INDIANAPOLIS, Feb. 21, 2019 /PRNewswire/ — A patient who received a first-in-the-world tricuspid valve procedure as part of a new national clinical trial at Franciscan Health Heart Center is home and recovering well. The procedure utilizes a revolutionary material called extracellular matrix. The material provides a seamless valve structure which engages and […]
$100 Million Invested in Clinical Research for Impella
DANVERS, Mass.–(BUSINESS WIRE)–In a milestone for scientific research, Abiomed (NASDAQ: ABMD) has now invested more than $100 million over the past five years in clinical research on the Impella heart pump platform. Abiomed’s commitment to clinical research is detailed on a new webpage that launched today. Abiomed-sponsored research is augmented by two decades of independent physician-led […]
ARCA Biopharma Announces FDA Agreement for a Single Phase 3 Clinical Trial to Support Approval for the First Genetically-Targeted Cardiovascular Drug
WESTMINSTER, Colo., Feb. 20, 2019 (GLOBE NEWSWIRE) — ARCA biopharma, Inc. (Nasdaq: ABIO), a biopharmaceutical company applying a precision medicine approach to developing genetically-targeted therapies for cardiovascular diseases, today announced that it has reached agreement with the U.S. Food and Drug Administration (FDA) regarding a Special Protocol Assessment (SPA) on the design of a […]
Edwards PASCAL Transcatheter System Receives CE Mark
IRVINE, Calif., Feb. 19, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced the Edwards PASCAL transcatheter valve repair system has received CE Mark for the treatment of patients with mitral regurgitation. “Mitral valve disease is complex, varied and […]
CHF Solutions Enters the South American Market with a Distribution Agreement with Bragenix LTDA in Brazil
EDEN PRAIRIE, Minn., Feb. 19, 2019 (GLOBE NEWSWIRE) — CHF Solutions (Nasdaq: CHFS), today announced the initiation of a distribution agreement in the Brazilian market with Bragenix, LTDA. This agreement expands the company’s worldwide sales outreach into South America by entering the largest market in the region. “We have aggressively […]
Final Patient Dosed in Mesoblast Phase 3 Trial of Revascor Cell Therapy for Advanced Heart Failure
NEW YORK and MELBOURNE, Australia, Feb. 19, 2019 (GLOBE NEWSWIRE) — Mesoblast Limited (Nasdaq:MESO; ASX:MSB), a world leader in development and commercialization of cellular medicines, announced today that the last patient has now been dosed in the Phase 3 events-driven trial of its allogeneic cell therapy product candidate Revascor for advanced chronic […]