LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN) (“LivaNova” or the “Company”), a market-leading medical technology company, today announced it received FDA 510(k) clearance for its MEMO 4D® semi-rigid mitral annuloplasty ring and confirmed the first implantation of the device. MEMO 4D, LivaNova’s next-generation of the MEMO device family, now offers a broader range of […]
Coronary/Structural Heart
Cardiovascular Research Consortium to Provide Access to Real-World Heart Care Data via the TriNetX Platform
CAMBRIDGE, Mass., June 12, 2018 /PRNewswire/ — The Cardiovascular Research Consortium (CRC), a nationwide network of community-based cardiology practices, has selected TriNetX’s real-world evidence platform to provide the biopharmaceutical industry with the first-of-its-kind access to extensive cardiology-specific clinical data. The data in combination with TriNetX’s advanced analytics capabilities and on-demand access, will enable […]
LivaNova’s Perceval Sutureless Aortic Heart Valve Approved in Japan
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN) (“LivaNova” or the “Company”), a market-leading medical technology company, today announced that Japan’s Ministry of Health, Labour and Welfare has approved the Company’s Perceval® sutureless aortic heart valve to treat aortic valve disease. “With this approval for Perceval, an innovative and trusted valve platform, we are able […]
Celixir announces US FDA approval of the IND application for cell therapy Heartcel
Stratford-upon-Avon, UK, 08 June 2018 – Celixir, a privately owned company discovering and developing life-saving advanced therapies, announces that the US Food and Drug Administration (FDA) has approved its Investigational New Drug application (IND) for HeartcelTM, its immuno-modulatory progenitor (iMP) cell therapy for the treatment of adult heart failure. Celixir announced […]
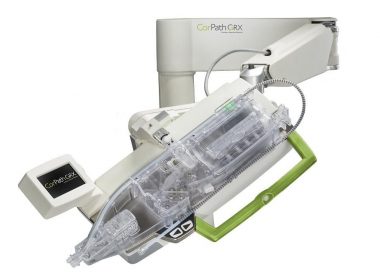
Corindus Announces Pharmaceutical and Medical Device Agency (PMDA) Approval of CorPath GRX System in Japan
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE American: CVRS], a leading developer of precision vascular robotics, announced today that it received Pharmaceutical and Medical Device Agency (PMDA) Approval for commercialization of its CorPath® GRX System in Japan. Japan is one of the largest markets in the world for percutaneous coronary interventions […]
Acasti Pharma to Present at the XVIII(th) International Symposium on Atherosclerosis
LAVAL, Québec, June 06, 2018 (GLOBE NEWSWIRE) — Acasti Pharma Inc. (NASDAQ:ACST) (TSX-V:ACST) (the “Company” or “Acasti Pharma”), a biopharmaceutical innovator focused on the research, development and commercialization of its prescription drug candidate CaPre® (omega-3 phospholipid) for the treatment of severe hypertriglyceridemia, today announced that the Company is scheduled to […]
Caladrius Biosciences Sells Rights to Counter-Flow Centrifugation System to Hitachi Chemical Advanced Therapeutics Solutions
BASKING RIDGE, N.J., June 05, 2018 (GLOBE NEWSWIRE) — Caladrius Biosciences, Inc. (Nasdaq:CLBS) (“Caladrius” or the “Company”), a clinical-stage biopharmaceutical company with multiple technology platforms targeting autoimmune and select cardiovascular indications, announces today that the Company has sold for $2.5 million its ownership interest in a development-stage, fully enclosed, automated, programmable […]
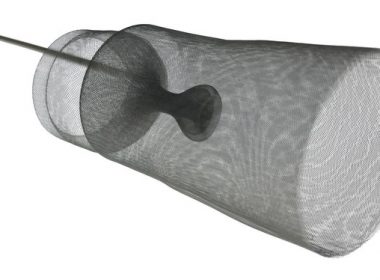
Emboline, Inc. Completes Initial Human Clinical Cases With The Emboliner™ Embolic Protection Catheter
SANTA CRUZ, Calif., June 5, 2018 /PRNewswire/ — Emboline, Inc., an emerging medical device company with a game-changing device for total embolic protection during transcatheter aortic valve replacement (TAVR) procedures, announced today the successful completion of its initial series of human clinical cases using the EmbolinerTM Embolic Protection Catheter. The initial procedures were performed at […]
Biomagnetic Imaging Company Genetesis Closes $7.5M in Series A Financing
MASON, Ohio–(BUSINESS WIRE)– Genetesis, a medical device company focused on using biomagnetic imaging to enable rapid, noninvasive and accurate chest pain triage, has closed an oversubscribed round of $7.5M in Series A financing. CincyTech led the round with participation from existing investors including Mark Cuban’s Radical Investments, and new investors including […]
TRiCares Closes €22 Million ($25.4 Million) Series B Financing Round to Fund Development of Minimally Invasive Treatment of Tricuspid Regurgitation
Paris, France and Munich, Germany, June 4, 2018 – TRiCares SAS, a privately held pioneer in the field of minimally invasive treatment of tricuspid regurgitation, is pleased to announce today the successful completion of a €22 Million ($25.4 Million) Series B financing round. Proceeds from the financing will be used to advance […]