CAMBRIDGE, Mass.–(BUSINESS WIRE)–Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY), the leading RNAi therapeutics company, announced today publication of data from exploratory cardiac assessments in the APOLLO Phase 3 study of patisiran, an RNAi therapeutic for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The results were published online today in the […]
Coronary/Structural Heart
FDA approves device for treatment of acute coronary artery perforations
SILVER SPRING, Md., Sept. 14, 2018 /PRNewswire/ — The U.S. Food and Drug Administration today approved a device intended to treat acute coronary artery perforations, or tears in the blood vessels of the heart. The PK Papyrus Covered Coronary Stent System is the first device approved by the FDA for this indication in […]
BioCardia Receives Two New U.S. Patents
SAN CARLOS, Calif., Sept. 14, 2018 /PRNewswire/ — BioCardia®, Inc. [OTC :BCDA ], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced the issuance of two United Statespatents. U.S. Patent No. 10,035,982 relates to methods of preparing culture-expanded cells for the treatment of heart failure. The patent has broad claims on […]
Infraredx to Present Results of the Lipid-Rich Plaque (LRP) Study at the Transcatheter Cardiovascular Therapeutics (TCT) 2018 Scientific Symposium
BURLINGTON, Mass.–(BUSINESS WIRE)–Infraredx, Inc., a pioneer in intravascular imaging for mapping coronary artery disease, today announced that results from the highly anticipated Lipid-Rich Plaque (LRP) Study have been accepted as a late-breaking clinical trial at the 30th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, to […]
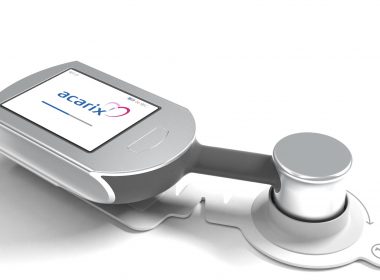
Acarix gains market acceptance in Austria; new customers orders CADScor®System for ruling out Coronary Artery Disease (CAD).
Press release Malmö, September 12, 2018 Acarix gains market acceptance in Austria; new customers orders CADScor®System for ruling out Coronary Artery Disease (CAD). Acarix AB (publ) (“Acarix”) reports first sales in Austria for its handheld CADScor®System for early rule-out of CAD. The Austrian sales mirror growing success in neighboring Germany […]
FDA to review supplemental Biologics License Application for Praluent® (alirocumab) Injection as potential treatment to reduce major adverse cardiovascular events
BRIDGEWATER, N.J. and TARRYTOWN, N.Y., Sept. 12, 2018 FDA also recently approved Praluent label update for some patients currently requiring LDL apheresis therapy /PRNewswire/ — Sanofi and Regeneron Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted a supplemental Biologics License Application (sBLA) for Praluent® (alirocumab) Injection, a PCSK9 inhibitor. The […]
MEDTRONIC STATEMENT REGARDING RECENT FDA WARNING LETTERS
DUBLIN – September 11, 2018 – Today, Medtronic issued the following statement confirming it has received FDA warning letters (CMS #560736 and #562437) related to its Cardiac Rhythm and Heart Failure facilities located in Mounds View, Minn., and in Juncos, Puerto Rico. The warning letters reflect earlier FDA inspection reports from […]
RenalGuard Solutions Debuts Newly Formed Reprieve Cardiovascular at Heart Failure Society of America Annual Meeting and Completes $7 Million Financing
MILFORD, Mass.–(BUSINESS WIRE)–Reprieve Cardiovascular™, a newly formed company affiliated with RenalGuard Solutions™, is a pioneering medical device company focused on improving outcomes for patients suffering from acute decompensated heart failure (ADHF). RenalGuard also announced the completion of a $7 million financing provided by strong support from existing investors and Abiomed, […]
CellAegis Announces Completion of Patient Enrollment in First North American Study for Innovative Heart Attack Therapy Using autoRIC®
TORONTO, Sept. 11, 2018 (GLOBE NEWSWIRE) — CellAegis Devices Inc. (“CellAegis” or the “Company”), a commercial-stage medical device company advancing innovative, non-invasive, safe and cost-effective solutions for acute and chronic cardiovascular conditions, today announced completion of patient enrollment in a large scale, investigator sponsored study (“FIRST”) to evaluate the clinical […]
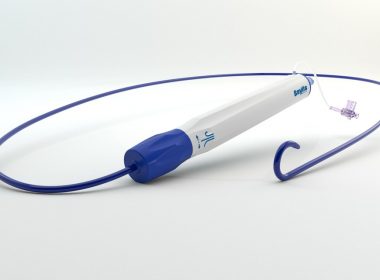
Baylis Medical Expands Transseptal Portfolio with Launch of Steerable Guiding Sheath
TORONTO, Sept. 10, 2018 /PRNewswire/ – Baylis Medical Company, a leader in the development, manufacturing and commercialization of cardiology devices, announced the official commercial launch of its SureFlex® Steerable Guiding Sheath. The SureFlex sheath is part of Baylis’ rapidly growing portfolio of high-performance products for transseptal access. The sheath features an advanced sheath-to-dilator […]