NEW YORK and MELBOURNE, Australia, July 17, 2018 (GLOBE NEWSWIRE) — Mesoblast Limited (Nasdaq:MESO) (ASX:MSB) today announced that it has entered into a strategic alliance with one of China’s largest pharmaceutical companies, Tasly Pharmaceutical Group (SHA: 600535; Tasly), for the development, manufacture and commercialization in China of Mesoblast’s allogeneic mesenchymal precursor cell […]
Coronary/Structural Heart
Polares Medical Closes a $25M Financing to Enter Clinical Validation
Lausanne, Switzerland and Palo Alto, USA, July 17, 2018 / B3C newswire / — Polares Medical SA, a preclinical stage medical technology company focused on the development of a unique trans-catheter mitral valve hemi-replacement system to treat patients suffering from mitral regurgitation (MR), announced today the closing of a $25 million […]
Reva Expands Commercial Access to Fantom With New Distribution Partnership in Italy
SAN DIEGO, July 15, 2018 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX:RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, is expanding commercial access to the Fantom® Bioresorbable Scaffold with a new distribution partnership in Italy. REVA will work with Bio Vascular Group S.R.L. (“Bio Vascular”) […]
CathWorks Receives CPT Code for New Wire-Free, Intraprocedural FFR Measurements to Optimize PCI Therapy Decisions
KFAR-SABA, Israel–(BUSINESS WIRE)–CathWorks announced the approval of a new CPT code 0523T for non-invasive, 3D FFRangio™ enabled interpretation of possible atherosclerotic stenosis during coronary angiography interventions. The code issuance is a major step forward in helping physicians objectively and cost-effectively determine if PCI (percutaneous coronary intervention) is indicated and revascularization […]
Abbott Receives FDA Approval for Next-Generation MitraClip® Device to Treat People with Leaky Heart Valves
ABBOTT PARK, Ill., July 12, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced it received approval from the U.S. Food and Drug Administration (FDA) for a next-generation version of its leading MitraClip® heart valve repair device used to repair a leaky mitral valve without open-heart surgery. The transcatheter clip-based therapy, now on a third generation […]
Hancock Jaffe Laboratories to Conduct Analysis for FDA Submission Package for Bioprosthetic Heart Valve
IRVINE, Calif., July 11, 2018 (GLOBE NEWSWIRE) — Hancock Jaffe Laboratories, Inc. (Nasdaq:HJLI) (Nasdaq:HJLIW), a company specializing in bioprosthetic medical devices to establish improved standards of care for treating cardiac and vascular diseases, today announced that it has initiated an analysis of its bioprosthetic heart valve in order to determine […]
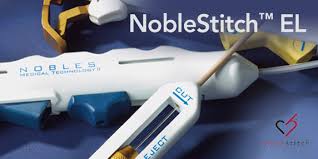
NobleStitch™ EL PFO Closure Performed Live For TVT/ PFO 2018 Congress
FRANKFURT, Germany, July 9, 2018 /PRNewswire/ — Prof. Dr. Achille Gaspardone, Director of Cardiology at Hospital of Sant’ Eugenio (Rome, Italy), and Prof. Dr. Horst Sievert MD, Director and Founder of Cardio Vascular Center (Frankfurt Germany), presented a LIVE PFO Closure to attendees of the Transcatheter Valve Therapies (TVT) Congress in Chicago, IL. The case was transmitted via […]
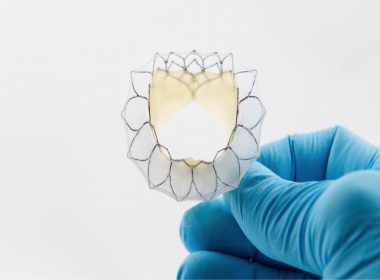
Morton Plant Hospital is First in Florida to Participate in Transcatheter Mitral Valve Replacement Pivotal Trial
CLEARWATER, Fla., July 2, 2018 /PRNewswire-USNewswire/ — On June 28, doctors at Morton Plant Hospital became the first in Florida to treat a patient in the APOLLO Transcatheter Mitral Valve Replacement (TMVR) Pivotal Trial. TMVR is a minimally invasive potential alternative to conventional open-heart mitral-valve surgery for patients with severe mitral valve regurgitation. Mitral valve regurgitation […]
Tiara and Reducer Featured at CSI Frankfurt 2018
VANCOUVER, July 2, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TZX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies, today announced that its Tiara™ (“Tiara”), a transcatheter treatment of mitral valve disease, and the Neovasc Reducer™ (“Reducer”), a CE-Marked medical devices used for […]
CHMP issues positive opinion to expand Jardiance®, Synjardy® and Glyxambi® labels to include positive effects on cardiovascular and renal outcomes
INGELHEIM, Germany & INDIANAPOLIS–(BUSINESS WIRE)–Boehringer Ingelheim and Eli Lilly and Company (NYSE:LLY) announced today that the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion to update the labels of Jardiance® (empagliflozin), Synjardy® (empagliflozin and metformin) and Glyxambi® (empagliflozin and linagliptin) to include additional important […]