KIRKLAND, Wash.–(BUSINESS WIRE)–Prevencio, Inc. today announces data which indicates its HART CAD and HART CVE tests accurately diagnose Coronary Artery Disease (CAD) and risk for Major Adverse Cardiac Events (MACE) in Diabetic Mellitus (DM) patients. Researchers believe the data, presented on June 24 at the American Diabetes Association 2018 Scientific Sessions, […]
Coronary/Structural Heart
Microbot Medical Strengthens Global IP with Issued Patent in India Covering the Company’s ViRob™ Technology Platform
HINGHAM, Mass., June 25, 2018 (GLOBE NEWSWIRE) — Microbot Medical Inc. (Nasdaq CM:MBOT), a medical device company specializing in the design and development of transformational micro-robotic medical technologies, today announced that Intellectual Property India has granted patent No. 296369, covering the Company’s ViRob™ technology platform. “This issued patent, coupled with […]
BIOLIFE4D Successfully Demonstrates Ability to 3D Bioprint Human Cardiac Tissue
CHICAGO, June 25, 2018 (GLOBE NEWSWIRE) — BIOLIFE4D, a biotech pioneer leveraging advances in tissue engineering to 3D print human organs viable for transplant, today announced it has successfully demonstrated its ability to 3D bioprint human cardiac tissue – specifically, a human cardiac patch. The scientific milestone was accomplished within days […]
Essential Medical Announces Positive Results From the SAFE MANTA Clinical Trial, the First Pivotal Trial for a Dedicated Large Bore Closure Device
EXTON, Pennsylvania, June 25, 2018 /PRNewswire/ — Essential Medical, Inc., a private medical device company focused on innovative solutions for large bore vascular closure, today announced the results from the SAFE MANTA IDE clinical trial, the first pivotal trial for a dedicated large bore closure device. Dr. David Wood, Co-Principal Investigator and founding […]
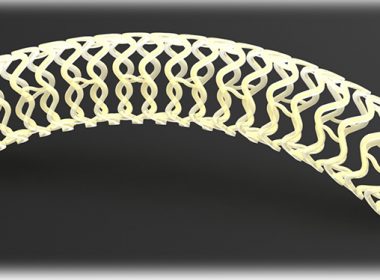
REVA Extends Technological Lead in Bioresorbable Scaffolds With CE Mark Approval of the Full Fantom® Encore Product Line
SAN DIEGO, June 18, 2018 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX:RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, today announced it has received CE Mark approval for its full Fantom® Encore product line. This covers Fantom Encore in the 3.0 and 3.5 mm diameters, […]
PlaqueTec Sponsors and Presents Data at Vulnerable Patient Meeting
CAMBRIDGE, England–(BUSINESS WIRE)–PlaqueTec announced today that it will sponsor the exclusive Vulnerable Patient Meeting (VPM), taking place 24-26 June in Stresa, Italy, for the fourth time. VPM, hosted by Cardialysis, is an annual invitation-only meeting focused on atherosclerosis for approximately 75-100 attendees, with a faculty of cardiology opinion leaders, regulators, […]
Idorsia initiates PRECISION – Phase 3 study with aprocitentan for resistant hypertension management
Allschwil, Switzerland – June 20, 2018 Idorsia Ltd (SIX: IDIA) today announced that the first patient has been enrolled into PRECISION, a Phase 3 study to investigate the efficacy and safety of aprocitentan for resistant hypertension management in adults. Hypertension, or high blood pressure, remains the most frequent addressable risk […]
Hancock Jaffe Laboratories Selects Site for First-in-Human VenoValve Study
IRVINE, Calif., June 20, 2018 (GLOBE NEWSWIRE) — Hancock Jaffe Laboratories, Inc. (Nasdaq:HJLI) (Nasdaq:HJLIW), a company specializing in bioprosthetic medical devices to establish improved standards of care for treating cardiac and vascular diseases, today announced that it has selected Fundación Santa Fe de Bogotá (“FSFB”), in Bogota Columbia, as the […]
Neovasc Announces First Implant of a Neovasc Reducer™ in a U.S. Patient
VANCOUVER, June 20, 2018 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies, today announced the first U.S. patient has been implanted with a Neovasc Reducer™ (the “Reducer”), a CE-Marked medical device for the treatment of refractory […]
JenaValve Technology Implants Initial Patients in CE Mark Study for the Treatment of Severe Aortic Regurgitation with Next-Generation TAVR System
IRVINE, Calif.–(BUSINESS WIRE)–JenaValve Technology, Inc., a developer and manufacturer of differentiated transcatheter aortic valve replacement (TAVR) systems, today announced initiation of patient enrollment and implantations associated with the CE Mark study of its next generation JenaValve Pericardial TAVR System using the CoronatixTM Transfemoral Delivery Catheter for the percutaneous treatment of patients […]