BVA and Pressure Session Attracted 400+ Clinicians; Duke University Showcased Valuable New BVA Findings in Dedicated Poster Presentation Oak Ridge, TN, April 02, 2025 (GLOBE NEWSWIRE) — Daxor Corporation (Nasdaq: DXR), the global leader in blood volume measurement technology, announces excellent visibility of Daxor’s technology at the premier global cardiology conference, the American College of Cardiology (ACC) 74th Annual Scientific Session and Expo held in Chicago from March 29-31st. New data presented from Duke University Medical Center confirmed blood volume analysis (BVA) as a more precise measure of congestion in heart failure patients along with a focus session on the value of BVA compared to less reliable pressure measurement in heart failure care. At a standing-room-only session attended by over 400 participants, titled, “When Pressure ≠ Volume: Understanding Complex Hemodynamic Situations and Pitfalls,” Dr. Marat Fudim of Duke University Health emphasized that pressure-based assessments frequently misrepresent true volume status. The research reinforces that volume management, rather than pressure management, should be the cornerstone of heart failure treatment. “The intense interest in the session highlights how much the awareness of BVA is growing and the need for direct volume measurement is being increasingly recognized in the cardiology community,” said Michael Feldschuh, Daxor’s President and CEO. New research findings were also showcased from the Duke study, “Correlation Between Changes in Total Blood Volume and Measures of Congestion During Heart Failure Hospitalization,” which revealed that the following commonly used proxy markers believed to be useful for volume care did not, in fact, correlate with intravascular volume: Urine outputWeight changeBiomarkers CA-125, NT–proBNP “This research from Duke adds to the substantial body of evidence of the unique value of BVA and reinforces the urgent need for precision in managing congestion in heart failure,” said John L. Jefferies, MD, MBA, MPH, Chief Medical Officer, Daxor Corporation. “Traditional methods often mislead, relying on indirect markers that do not accurately reflect true blood volume status. BVA provides the clarity clinicians need to make informed decisions, optimize treatment, and ultimately improve patient outcomes.” About ACC The American College of Cardiology (ACC) envisions a world where science, knowledge and innovation optimize cardiovascular care and outcomes. We believe in the power of community. With more than 56,000 members worldwide spanning the entire cardiovascular team, we serve as the professional home for clinicians and researchers seeking the latest science, research, and education. United with our members, chapters, and global cardiovascular partners, we are focused on transforming cardiovascular care and improving heart health for all. For more information visit https://www.acc.org/. About Daxor Corporation Daxor Corporation (Nasdaq: DXR), is the global leader in blood volume measurement technology focused on blood volume testing innovation. We developed and market the BVA-100® (Blood Volume Analyzer), the only diagnostic blood test cleared by the FDA to provide safe, accurate, objective quantification of blood volume status and composition compared to patient-specific norms. Over 65,000+ tests have been performed at leading hospital centers across the U.S., enhancing hospital performance metrics in a broad range of surgical and medical conditions, including significantly reducing mortality and readmissions in heart failure and critical care. Daxor has several ongoing trials in the areas of heart failure treatment with support from the NIH and is under contract developing analyzers to improve combat casualty care with the U.S. Department of Defense. Daxor’s mission is to advance healthcare by enabling optimal fluid management with blood volume analysis. Daxor’s vision is optimal blood volume for all. For more information, please visit our website at Daxor.com. Sign up to receive news on Daxor’s innovative technology HERE. Forward-Looking Statements Certain statements in this release may include forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including without limitation statements regarding the impact of hiring sales staff and expansion of our distribution channels. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in this release, including, without limitation, those risk associated with our post-market clinical data collection activities, benefits of our products to patients, our expectations with respect to product development and commercialization efforts, our ability to increase market and physician acceptance of our products, potentially competitive product offerings, intellectual property protection, FDA regulatory actions, our ability to integrate acquired businesses, our expectations regarding anticipated synergies with and benefits from acquired businesses, and additional other risks and uncertainties described in our filings with the SEC. Forward-looking statements speak only as of the date when made. Daxor does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Investor Relations Contact:Bret ShapiroSr. Managing Partner, CORE IR1-516-222-2560brets@coreir.com
Coronary/Structural Heart
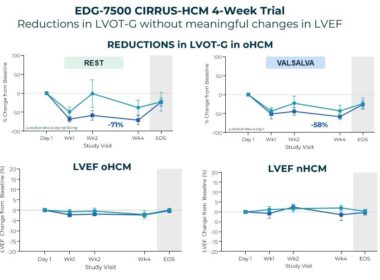
Edgewise Therapeutics Announces Positive Top-Line Results from Phase 2 CIRRUS-HCM Four-Week Trial of EDG-7500 in Hypertrophic Cardiomyopathy (HCM)
– Phase 2 trial of EDG-7500 demonstrated rapid and clinically meaningful reductions in LVOT gradients in participants with obstructive HCM – – Four-week treatment with EDG-7500 demonstrated substantial improvements in measures of feel and function, reductions in key cardiac biomarkers and…
Morton Plant Hospital First in World to Perform TAV Replacement in RESTORE Study
CLEARWATER, Fla., April 1, 2025 /PRNewswire/ — BayCare’s Morton Plant Hospital in Clearwater recently completed the world’s first TAV-in-TAV as part of a study called RESTORE. TAV-in-TAV is for people who have undergone a prior TAVR procedure, but the replacement valve is coming to the…
Medera Presented Updated Results from First-In-Human Gene Therapy Trial for Heart Failure with Preserved Ejection Fraction at 2025 HFpEF Summit
BOSTON, April 01, 2025 (GLOBE NEWSWIRE) — Medera Inc. (“Medera”), a clinical-stage biopharmaceutical company focused on targeting cardiovascular diseases by developing a range of next-generation therapeutics, today announced that updated results from its ongoing MUSIC-HFpEF Phase 1/2a clinical trial investigating SRD-002, a one-time gene therapy treatment delivered through a proprietary minimally invasive intracoronary infusion methodology, were presented at the 2025 HFpEF Summit held March 19-21, 2025, in Beverly Hills, CA. Heart failure is a global pandemic with an estimated 64.3 million cases worldwide, costing over US$100B per year. Heart failure with preserved ejection fraction (HFpEF) accounts for nearly half of all heart failure cases, but has limited disease-modifying therapeutics. SRD-002 is a gene therapy utilizing an adeno-associated type 1 virus vector carrying the cardiac isoform of the sarcoplasmic reticulum calcium ATPase pump (SERCA2a) that directly targets the molecular pathways underlying the core pathology of HFpEF by enhancing myocardial relaxation and reducing stiffness. The presentation titled, “Targeting Diastolic Dysfunction in HFpEF- SERCA2a Gene Therapy,” was delivered by Marat Fudim, MD, MHS, Advanced Heart Failure Specialist and Associate Professor at Duke University Medical Center and site principal investigator, on Friday, March 21st. The presentation highlighted data from the trial, which, as of the data cutoff date of February 25, 2025, has treated five patients in Cohort A with a low dose of 3×10¹³ viral genomes (vg) per patient and one patient has been dosed in Cohort B at a dose of 4.5×10¹³ vg per patient. With follow-up ranging from 2 to 15 months, no gene therapy-related serious adverse events have been reported. Four out of the five patients in the low-dose group have shown improvements in New York Heart Association (NYHA) heart failure classification at 6 months, with clinically meaningful improvements in 6-minute walk test (6MWT), decreases/stabilization in NT-Pro-BNP, and high-sensitivity troponin observed in some patients. The enrollment of patients at the higher dose of 4.5×1013 vg per patient is ongoing. “New treatment approaches, like Medera’s gene therapy product, targeting critical pathways in the heart, are crucial for patients and caregivers faced with this difficult-to-treat disease,” stated Marat Fudim, MD, MHS, Advanced Heart Failure Specialist and Associate Professor at Duke University Medical Center and site principal investigator. “The encouraging early results presented at this summit reflect important progress in this critical research.” “We are excited to share our encouraging results from the MUSIC-HFpEF trial at this prestigious summit,” said Ronald Li, Ph.D., CEO and co-founder of Medera. “This groundbreaking trial represents the first-in-human gene therapy approach for patients with HFpEF and features unique hemodynamic characterization of patients within the study using precise measurements of pulmonary capillary wedge pressure (PCWP) at rest and during exercise. Our clinical findings suggest this approach may offer an alternative treatment strategy to HFpEF patients, where a significant unmet need remains.” The HFpEF Summit is a biannual, two-day meeting bringing together internationally recognized leaders in the clinical, biological, and translational study of HFpEF. This summit focused on the latest insights and innovations for scientists, clinicians, researchers, and other healthcare professionals interested in all aspects of HFpEF. For additional information about the MUSIC-HFpEF trial, visit ClinicalTrials.gov using the study identifier NCT06061549. On September 5, 2024, Medera and Keen Vision Acquisition Corporation (“KVAC”) (NASDAQ:KVAC, KVACW), announced they had entered into a definitive merger agreement. About Heart Failure with Preserved Ejection Fraction (HFpEF) Heart failure (HF) is a global pandemic with an estimated 64.3 million cases worldwide and a rising prevalence trend. Accounting for 50% or more of the overall HF population, HFpEF is an age-related condition that has become increasingly prevalent in recent years. This surge is partly due to better awareness and identification of the condition and partly due to lifestyle changes affecting cardiac myocytes. Individuals affected by HFpEF experience similar morbidity and mortality to patients with HF with reduced ejection fraction (HFrEF). Despite the growing epidemic of this emerging syndrome, HFpEF-focused interventional trials have had little success, except for the use of sacubitril-valsartan (Entresto™) and the sodium glucose transporter-2 (SGLT-2) inhibitor empagliflozin (Jardiance™) for reducing cardiovascular mortality and heart failure hospitalization. However, these agents are not disease-modifying, highlighting the critical need for therapeutic interventions targeting the physiological mechanisms involved in HFpEF. About Medera Medera is a clinical-stage biopharmaceutical company focused on targeting difficult-to-treat and currently incurable diseases by developing a range of next-generation therapeutics. Medera operates via its two preclinical and clinical business units, Novoheart and Sardocor, respectively. Novoheart capitalizes on the world’s first and award-winning “mini-Heart” Technology for revolutionary disease modelling and drug discovery, uniquely enabling the modelling of human-specific diseases and discovery of therapeutic candidates free from species-specific differences in accordance to the FDA Modernization Act 2.0. Novoheart’s versatile technology platform provides a range of state-of-the-art automation hardware and software as well as screening services, for human-specific disease modelling, therapeutic target discovery and validation, drug toxicity and efficacy screening, and dosage optimization carried out in the context of healthy and/or diseased human heart chambers and tissues. Global pharmaceutical and academic leaders are using Novoheart’s technology platform for their drug discovery and development purposes. The Novoheart platform has facilitated and accelerated the development of Sardocor’s lead therapeutic candidates that are currently in clinical trials. Sardocor is dedicated to the clinical development of novel next-generation therapies for Medera. Leveraging Novoheart’s human-based drug discovery and validation platforms, Sardocor aims to expedite drug development and regulatory timelines for its gene and cell therapy pipeline. Sardocor has received Investigational New Drug (IND) clearances from the FDA for three ongoing AAV-based cardiac gene therapy clinical trials targeting Heart Failure with Reduced Ejection Fraction (HFrEF), Heart Failure with Preserved Ejection Fraction (HFpEF) with the Fast Track Designation, and Duchenne Muscular Dystrophy-associated Cardiomyopathy (DMD-CM) with the Orphan Drug Designation. Additionally, Sardocor’s pipeline includes four preclinical gene therapy and three preclinical small molecule candidates targeting various cardiac, pulmonary, and vascular diseases. For more information, please visit www.medera.bio. About Keen Vision Acquisition Corporation Keen Vision Acquisition Corp (“KVAC”), listed on Nasdaq, is a blank check company incorporated for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses or entities. KVAC is focused on biotechnology, consumer goods or agriculture opportunities, which are also evaluated on their sustainability, environmental, social, and corporate governance (“ESG”) imperatives. For more information, please visit www.kv-ac.com. Forward-Looking Statements Certain statements included in this press release are not historical facts but are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this press release are forward-looking statements. Any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are also forward-looking statements. In some cases, you can identify forward-looking statements by words such as “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “strategy,” “future,” “opportunity,” “may,” “target,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” “preliminary,” or similar expressions that predict or indicate future events or trends or that are not statements of historical matters, but the absence of these words does not mean that a statement is not forward-looking. Forward-looking statements include, without limitation, KVAC’s, Medera’s, or their respective management teams’ expectations concerning the outlook for their or Medera’s business, productivity, plans, and goals for future operational improvements and capital investments, operational performance, future market conditions, or economic performance and developments in the capital and credit markets and expected future financial performance, including expected net proceeds, expected additional funding, the percentage of redemptions of KVAC’s public shareholders, growth prospects and outlook of Medera’ operations, individually or in the aggregate, including the achievement of project milestones, commencement and completion of commercial operations of certain of Medera’s projects, as well as any information concerning possible or assumed future results of operations of Medera. Forward-looking statements also include statements regarding the expected benefits of the transactions contemplated by the merger (“Transaction”). The forward-looking statements are based on the current expectations of the respective management teams of Medera and KVAC, as applicable, and are inherently subject to uncertainties and changes in circumstance and their potential effects. There can be no assurance that future developments will be those that have been anticipated. These forward-looking statements involve a number of risks, uncertainties or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, (i) the risk that the Transaction may not be completed in a timely manner or at all, which may adversely affect the price of KVAC’s securities; (ii) the risk that the Transaction may not be completed by KVAC’s business combination deadline and the potential failure to obtain an extension of the business combination deadline if sought by KVAC; (iii) the failure to satisfy the conditions to the consummation of the Transaction, including the adoption of the Merger Agreement by the shareholders of KVAC and the receipt of certain regulatory approvals; (iv) market risks; (v) the occurrence of any event, change or other circumstance that could give rise to the termination of the Merger Agreement; (vi) the effect of the announcement or pendency of the Transaction on Medera’s business relationships, performance, and business generally; (vii) the outcome of any legal proceedings that may be instituted against Medera or KVAC related to the Merger Agreement or the Transaction; (viii) failure to realize the anticipated benefits of the Transaction; (ix) the inability to maintain the listing of KVAC’s securities or to meet listing requirements and maintain the listing of Medera’s securities on Nasdaq; (x) the inability to implement business plans, forecasts, and other expectations after the completion of the Transaction, identify and realize additional opportunities, and manage its growth and expanding operations; (xi) risks related to Medera’s ability to develop, license or acquire new therapeutics; (xii) the risk that Medera will need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; (xiii) the risk of product liability or regulatory lawsuits or proceedings relating to Medera’s business; (xiv) uncertainties inherent in the execution, cost, and completion of preclinical studies and clinical trials; (xv) risks related to regulatory review, and approval and commercial development; (xvi) risks associated with intellectual property protection; (xvii) Medera’s limited operating history and risk that it may never successfully commercialise its products; (xviii) Medera expects to continue to incur significant losses and may never achieve or maintain profitability; and (xix) the risk that additional financing in connection with the Transaction may not be raised on favorable terms. The foregoing list is not exhaustive, and there may be additional risks that neither KVAC nor Medera presently knows or that KVAC and Medera currently believe are immaterial. You should carefully consider the foregoing factors, any other factors discussed in this press release and the other risks and uncertainties described in the “Risk Factors” section of KVAC’s Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the SEC on March 29, 2024, the risks to be described in the registration statement, which will include a preliminary proxy statement/prospectus, and those discussed and identified in filings made with the SEC by KVAC from time to time. Medera and KVAC caution you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth in this press release speak only as of the date of this press release. Neither Medera nor KVAC undertakes any obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs. In the event that any forward-looking statement is updated, no inference should be made that Medera or KVAC will make additional updates with respect to that statement, related matters, or any other forward-looking statements. Any corrections or revisions and other important assumptions and factors that could cause actual results to differ materially from forward-looking statements, including discussions of significant risk factors, may appear, up to the consummation of the Transaction, in KVAC’s public filings with the SEC, and which you are advised to review carefully. Important Information for Investors and Shareholders In connection with the Transaction, KVAC and Medera filed a registration statement with the SEC, which includes a prospectus with respect to the securities to be issued in connection with the Transaction and a proxy statement to be distributed to holders of KVAC’s ordinary shares in connection with KVAC’s solicitation of proxies for the vote by KVAC’s shareholders with respect to the Transaction and other matters to be described in the Registration Statement (the “Proxy Statement”). After the SEC declares the registration statement effective, KVAC plans to mail copies to shareholders of KVAC as of a record date to be established for voting on the Transaction. This press release does not contain all the information that should be considered concerning the Transaction and is not a substitute for the registration statement, Proxy Statement or for any other document that KVAC may file with the SEC. Before making any investment or voting decision, investors and security holders of KVAC are urged to read the registration statement and the Proxy Statement, and any amendments or supplements thereto, as well as all other relevant materials filed or that will be filed with the SEC in connection with the Transaction as they become available because they will contain important information about, Medera, KVAC and the Transaction. Investors and security holders will be able to obtain free copies of the registration statement, the Proxy Statement and all other relevant documents filed or that will be filed with the SEC by KVAC through the website maintained by the SEC at www.sec.gov. In addition, the documents filed by KVAC may be obtained free of charge from KVAC’s website at https://www.kv-ac.com or by directing a request to info@kv-ac.com. The information contained on, or that may be accessed through, the websites referenced in this press release is not incorporated by reference into, and is not a part of, this press release. Participants in the Solicitation KVAC, Medera and their respective directors, executive officers and other members of management and employees may, under the rules of the SEC, be deemed to be participants in the solicitations of proxies in connection with the Transaction. For more information about the names, affiliations and interests of KVAC’s directors and executive officers, please refer to KVAC’s annual report on Form 10-K filed with the SEC on March 29, 2024, which can be found at https://www.sec.gov/ix?doc=/Archives/edgar/data/1889983/000121390024027973/ea0201104-10k_keenvision.htm and registration statement, Proxy Statement and other relevant materials filed with the SEC in connection with the Transaction when they become available. Additional information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, which may, in some cases, be different than those of KVAC’s shareholders generally, will be included in the registration statement and the Proxy Statement and other relevant materials when they are filed with the SEC when they become available. Shareholders, potential investors and other interested persons should read the registration statement and the Proxy Statement and other such documents carefully, when they become available, before making any voting or investment decisions. You may obtain free copies of these documents from the sources indicated above. No Offer or Solicitation This communication shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities in the Transaction shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Contacts:Keen Vision Acquisition CorporationAlex DavidkhanianChief Financial OfficerEmail: alex.davidkhanian@kv-ac.com MederaInvestor RelationsStephanie CarringtonICR HealthcareStephanie.Carrington@icrhealthcare.com(646) 277-1282 Media RelationsSean LeousICR HealthcareSean.Leous@icrhealthcare.com(646) 866-4012
Attralus Presents New Data on Its Pan-Amyloid Diagnostic Candidates at the 2025 American College of Cardiology (ACC) Annual Scientific Session
NAPLES, Fla., April 01, 2025 (GLOBE NEWSWIRE) — Attralus, Inc., a clinical stage biopharmaceutical company developing transformative medicines and diagnostics to improve the lives of patients with systemic amyloidosis, announced three poster presentations (from four investigator-initiated trials), on the use of 124I-evuzamitide, the company’s pan-amyloid binding imaging agent in development for the diagnosis of cardiac amyloidosis, and one poster presentation of encouraging new clinical data from the University of Tennessee Graduate School of Medicine from an investigator-initiated trial on AT-05 (99mTc-p5+14) for the diagnosis of cardiac amyloid, at the 2025 American College of Cardiology (ACC) Annual Scientific Session held in Chicago, IL on March 29-April 1, 2025.
Teleflex Receives FDA 510(k) Clearance of the AC3 Range™ Intra-Aortic Balloon Pump (IABP)
The addition to the IABP family supports uncompromised cardiac support for patients during transport The addition to the IABP family supports uncompromised cardiac support for patients during transport
New Data Confirm Rapid, Unpredictable Progression of Severe Aortic Stenosis and Need for Urgent Referral and Evaluation of Patients
CHICAGO–(BUSINESS WIRE)– Edwards Lifesciences (NYSE: EW) today announced new scientific evidence presented and published during the American College of Cardiology’s (ACC) Annual Scientific Session & Expo, addressing the critical needs of patients with structural heart disease. Without treatment, 1 in 10 patients experiencing symptoms of severe aortic stenosis (AS) may […]
Cleerly Unveils Groundbreaking Late-Breaking Clinical Trial Results Demonstrating AI-QCT’s Predictive Power for Women’s Cardiovascular Risk at ACC.25
New Findings from the CONFIRM2 Registry Reveal Significant Gender Disparities in Coronary Plaque Features and Associated Risks for Major Adverse Cardiovascular Events March 31, 2025 (DENVER) – Cleerly, the leader in cardiovascular AI imaging, has announced revolutionary findings from its late-breaking clinical trial presented at the American College of […]
Tenaya Therapeutics Announces Late Breaker Presentation of New Data from MyPEAK™-1 Phase 1b/2 Clinical Trial of TN-201 at American College of Cardiology Annual Meeting
TN-201 Has Been Well Tolerated at 3E13 vg/kg Dose New Biopsy Data Reaffirm Robust Transduction and RNA Expression with TN-201; RNA and Protein Levels Increase Over Time All Cohort 1 Patients with Severe Disease at Baseline Achieved NYHA Class I Two of Three Patients Experienced Improvements in One or More Measures of Hypertrophy Expects to Complete Enrollment of Cohort 2 in 1H25 and to Report Initial Data in 2H25 SOUTH SAN FRANCISCO, Calif., March 31, 2025 (GLOBE NEWSWIRE) — Tenaya Therapeutics, Inc. (NASDAQ: TNYA), a clinical-stage biotechnology company with a mission to discover, develop and deliver potentially curative therapies that address the underlying causes of heart disease, announced that interim data from the first three patients enrolled in the company’s MyPEAK-1 Phase 1b/2 clinical trial of TN-201 were highlighted in a Late-Breaker presentation at the 2025 American College of Cardiology Scientific Sessions (ACC.25). TN-201 is being developed for the potential treatment of Myosin Binding Protein C3 MYBPC3-associated hypertrophic cardiomyopathy (HCM), a condition caused by insufficient levels of myosin-binding protein C (MyBP-C). “TN-201 is the first gene therapy to be tested in HCM patients whose disease is caused by mutations to the MYBPC3 gene. For patients with this mutation, their disease is often more aggressive in its progression and they are at higher risk of serious – sometimes fatal – complications,” said Milind Desai, M.D., M.B.A, Haslam Family Endowed Chair in Cardiovascular Medicine, Vice Chair of Education in the Heart Vascular Thoracic Institute, Director of the Hypertrophic Cardiomyopathy Center at Cleveland Clinic, and an investigator for the MyPEAK-1 Phase 1b/2 clinical trial. “I look forward to continuing to study and evaluate this to see if this patient population is one that could be treated with gene therapy, as we have seen with other diseases.” “TN-201’s emerging safety profile at the 3E13 vg/kg dose, combined with biopsy results showing robust transduction, encouraging expression in the heart and suggestions of clinical improvement or stability are positive early signals of TN-201’s clinical impact,” said Whit Tingley, M.D., PhD., Tenaya’s Chief Medical Officer. “These data reinforce our optimism about TN-201’s potential to transform the treatment landscape for MYBPC3-associated HCM patients by addressing the underlying cause of their disease. We look forward to reporting additional data from Cohort 1 and getting our first look at the higher dose cohort later this year. We are profoundly grateful to the HCM community for their support of this work, and particularly to all those participating in the MyPEAK-1 clinical trial.” Interim data presented at ACC.25 include results from serial biopsies and assessments of Patient 1 and 2 at Week 52 and Patient 3 at Week 26, analyzing changes over time in the first three patients to receive a one-time infusion of TN-201 gene therapy (Cohort 1). TN-201 was generally well tolerated at 3E13 vg/kg, and treatment-emergent adverse events (AEs) were primarily mild, manageable and/or reversible.Serial biopsies taken at two timepoints for all three patients demonstrated sustained presence of TN-201 DNA in the heart (0.8 to 1.4 vg/dg) and robust TN-201 RNA expression ( >1.25×105 copies per microgram of RNA) that increased as much as 13-fold from Week 8 to Week 52 post-dose.MyBP-C protein levels increased from 56 to 59% and from 62 to 64% of normal between Week 8 and Week 52 for Patients 1 and 2, respectively. This increase, combined with the increase observed in TN-201 mRNA expression, suggest that TN-201 gene therapy is successfully being transcribed and expressed after reaching target cells. Patient 3 was the first patient on study to receive a baseline biopsy, which is expected to offer insight into the total change in protein levels following TN-201 treatment. The post-dose biopsy sample from Patient 3 was not evaluable; a second post dose biopsy is planned later this year and will be reported in a future data readout. Cardiac troponin, a biomarker of myocardial injury, was elevated in Cohort 1 patients at baseline and decreased by more than 60% in two patients, whose levels are now normal or near normal. NT-proBNP, a biomarker of cardiac strain, increased and remained elevated while patients were on immunosuppression, but returned to baseline as immunosuppressive drugs were discontinued.Key measures of hypertrophy, or enlargement of the heart, improved in two patients while other assessments remained stable. Left ventricular posterior wall thickness, which was elevated at baseline, decreased in two patients by up to 40% into the normal range for healthy individuals. In one patient, left ventricular mass (LVMI) improved by 10%. Additional measures of hypertrophy and diastolic function remained stable.All three patients in Cohort 1 had objectively severe disease at the time of enrollment with mild-to moderate heart failure symptoms (New York Heart Association (NYHA) classification II or III) that interfered in activities of daily living. All three have now achieved NYHA Class I, defined as having no limitations on physical activity. “We are excited by these promising early data from Dose Cohort 1 and the prospects for initial data from Dose Cohort 2 later this year where two of three patients have already been dosed,” said Faraz Ali, Chief Executive Officer of Tenaya. “We are also pleased that with our recent financing, plus adjustments to our spend, we have updated our cash guidance into the second half of 2026 and are well-positioned to achieve important clinical data milestones on both the TN-201 and TN-401 gene therapy programs over the next 12 months.” The interim MyPEAK-1 data were presented today by Dr. Desai during the Clinical and Investigative Horizons session in a Late-breaker presentation talk titled, “First Report of Phase 1b/2a Study Evaluating Safety and Early Efficacy of TN-201, an Adeno-Associated Virus Serotype 9 Gene Replacement Therapy, in Adults with MYBPC3-Associated Hypertrophic Cardiomyopathy”. Tenaya researchers also presented data at ACC.25 offering new insights into the disease burden of patents with MYBPC3-associated HCM compared to other HCM populations. Results from this study, “Differences in Patient Characteristics and Burden of Disease in Adults with MYBPC3-Associated HCM (#129)”, conducted in collaboration with the Sarcomeric Human Cardiomyopathy Registry (SHaRE), analyzed outcomes for 1,637 MYBPC3-associated HCM adults by age of diagnosis. Patients with pathogenic/likely pathogenic MYBPC3 mutations were found to be at risk for serious clinical manifestations including heart failure, arrhythmias, and sudden cardiac death.Younger patients progressed more rapidly and were more likely to experience serious outcomes.Approximately 50% of adult patients diagnosed before the age of 40 experience a serious cardiac event by the age of 50. Both presentations are available on the Tenaya website. Cohorts 1 and 2 in the Ongoing MyPEAK-1 Phase 1b/2 Clinical TrialData reported today focus on changes over time in the first three patients to receive TN-201 gene therapy. All three participants presented with nonobstructive HCM having previously undergone myectomy and remained at sufficiently high risk of sudden cardiac death to warrant an implantable cardiac defibrillator device (ICD). Per protocol, all patients received immunosuppressives consisting of sirolimus and prednisone before and after dosing, which was tapered over time based on monitoring of liver and inflammatory markers. All three patients have successfully tapered off immunosuppressives and remain on study. Following dosing of the first three patients in MyPEAK-1, an independent data and safety monitoring board (DSMB) evaluated available safety data. The DSMB endorsed the broadening of eligibility criteria and enrollment of the planned high dose cohort. Accordingly, patients are now being enrolled in Cohort 2 to receive TN-201 at a dose of 6E13 vg/kg. Tenaya anticipates completion of enrollment of the first three patients in Cohort 2 in the first half of the year and plans to share initial safety and biopsy data from Cohort 2, along with additional follow-up data from Cohort 1, in the second half of this year. About the MyPEAK-1 Phase 1b/2 Clinical TrialThe MyPEAK-1 Phase 1b/2 clinical trial (Clinicaltrials.gov ID: NCT05836259) is an ongoing, multi-center, open-label, dose-escalating study designed to assess the safety, tolerability and clinical efficacy of a one-time intravenous infusion of TN-201 gene replacement therapy. The trial is enrolling symptomatic (New York Heart Association Class II or III) adults who have been diagnosed with MYBPC3-associated HCM. MyPEAK-1 is testing doses of 3E13 vg/kg and 6E13 vg/kg in two cohorts of three patients each. MyPEAK-1 may enroll up to 24 MYBPC3-associated HCM adults with either nonobstructive or obstructive forms of HCM in planned dose expansion cohorts. To learn more about gene therapy for HCM and participation in the MyPEAK-1 study, please visit HCMStudies.com. About MYBPC3-Associated Hypertrophic Cardiomyopathy Variants in the Myosin Binding Protein C3 (MYBPC3) gene are the most common genetic cause of hypertrophic cardiomyopathy (HCM), accounting for approximately 20% of the overall HCM population, or 120,000 patients, in the United States alone.(1) MYBPC3-associated HCM is a severe and progressive condition affecting adults, teens, children and infants. Mutations of the MYBPC3 gene result in insufficient expression of a protein, called MyBP-C, needed to regulate heart contraction. The heart becomes hypercontractile and the left ventricle thickens, resulting in symptoms such as chest pain, shortness of breath, palpitations and fainting. Patients whose disease is caused by MYBPC3 mutations are more likely than those with non-genetic forms of HCM to experience earlier disease onset and have high rates of serious outcomes, including heart failure symptoms, arrhythmias, stroke and sudden cardiac arrest or death.(2) There are currently no approved therapeutics that address the underlying genetic cause of HCM. About TN-201TN-201 is an adeno-associated virus serotype 9 (AAV9)-based gene therapy designed to deliver a working MYBPC3 gene to heart muscle cells via a single intravenous infusion, increasing MyBP-C protein levels to address the underlying cause of MYBPC3-associated HCM with the aim of halting or even reversing disease after a single dose. The U.S. Food and Drug Administration has granted TN-201 Fast Track, Orphan Drug and Rare Pediatric Drug Designations. TN-201 has also received orphan medicinal product designation from the European Commission. About Tenaya TherapeuticsTenaya Therapeutics is a clinical-stage biotechnology company committed to a bold mission: to discover, develop and deliver potentially curative therapies that address the underlying drivers of heart disease. Tenaya employs a suite of integrated internal capabilities, including modality agnostic target validation, capsid engineering and manufacturing, to generate a portfolio of genetic medicines aimed at the treatment of both rare genetic disorders and more prevalent heart conditions. Tenaya’s pipeline includes TN-201, a gene therapy for MYBPC3-associated hypertrophic cardiomyopathy (HCM), TN-401, a gene therapy for PKP2-associated arrhythmogenic right ventricular cardiomyopathy (ARVC), TN-301, a small molecule HDAC6 inhibitor intended for heart failure with preserved ejection fraction (HFpEF), and multiple early-stage programs in preclinical development. For more information, visit www.tenayatherapeutics.com. (1) Sedaghat-Hemedani, et al., Clinical Research Cardiology, 2017(2) Ho, et al., Circulation 2018 Forward Looking Statements This press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Words such as “expects,” “potential,” “look forward,” “optimism,” “could,” “encouraging,” “planned,” “guidance” and similar expressions are intended to identify forward-looking statements. Such forward-looking statements include, among other things, the planned timing to complete enrollment and report additional data from MyPEAK-1; the clinical, therapeutic and commercial potential of, and expectations regarding TN-201 as a treatment for MYBPC3-associated HCM; the value of additional MyPEAK-1 data to inform the potential of TN-201; the inferences regarding MyBP-C protein and mRNA expression; statements regarding the continued development TN-201 and TN-201 clinical outcomes, which may materially change as patient enrollment continues or more patient data become available; Tenaya’s cash guidance commitment to focusing its resources on generating clinical data for its gene therapy pipeline and statements made by Tenaya’s Chief Medical Officer and Chief Executive Officer and investigator for MyPEAK-1. The forward-looking statements contained herein are based upon Tenaya’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. These forward-looking statements are neither promises nor guarantees and are subject to a variety of risks and uncertainties, including but not limited to: availability of MyPEAK-1 data at the referenced time; the timing and progress of MyPEAK-1; the potential failure of TN-201 to demonstrate safety and/or efficacy in clinical testing; the potential for any MyPEAK-1 clinical trial results to differ from preclinical, interim, preliminary or expected results; Tenaya’s ability to enroll and maintain patients in clinical trials, including MyPEAK-1; risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and operating as an early stage company; Tenaya’s continuing compliance with applicable legal and regulatory requirements; Tenaya’s ability to raise any additional funding it will need to continue to pursue its product development plans; Tenaya’s reliance on third parties; Tenaya’s manufacturing, commercialization and marketing capabilities and strategy; the loss of key scientific or management personnel; competition in the industry in which Tenaya operates; Tenaya’s ability to obtain and maintain intellectual property protection for its product candidates; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section titled “Risk Factors” in Tenaya’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and other documents that Tenaya files from time to time with the Securities and Exchange Commission. These forward-looking statements are made as of the date of this press release, and Tenaya assumes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. ContactMichelle CorralVP, Corporate Communications and Investor RelationsIR@tenayathera.com InvestorsAnne-Marie FieldsPrecision AQ (formerly Stern Investor Relations)annemarie.fields@precisionaq.com Media Wendy Ryan Ten Bridge Communications wendy@tenbridgecommunications.com
Acoramidis Shows Statistically Significant Improvements in Cardiovascular Outcomes in Patients with Variant ATTR-CM
Acoramidis’s hazard ratio of .41 for time to ACM or first CVH versus placebo in the ATTRibute-CM study subgroup of ATTRv-CM patients achieved statistical significance in a pre-specified analysisThis profound treatment effect, due to the near-complete stabilization (≥90%) and binding of TTR, represents the greatest observed benefit to date for ATTRv-CM patients, an ATTR-CM population with poor prognosis In the ATTRibute-CM study, acoramidis demonstrated the most rapid benefit seen in any Phase 3 study of ATTR-CM to date in both ATTRv-CM and ATTRw-CM patients: In as few as 3 months, the time to first event (ACM or CVH) durably separated relative to placeboA 42% reduction in composite ACM and recurrent CVH events relative to placebo at Month 30A 50% reduction in the cumulative frequency of CVH events relative to placebo at Month 30 Acoramidis is approved as Attruby™ by the U.S. FDA and is approved as BEYONTTRA™ by the European Commission and Japanese Pharmaceuticals and Medical Devices Agency PALO ALTO, Calif., March 31, 2025 (GLOBE NEWSWIRE) — BridgeBio Pharma, Inc. (Nasdaq: BBIO) (“BridgeBio” or the “Company”), a new type of biopharmaceutical company focused on genetic diseases, today presented results showing statistically significant improvements in clinical outcomes as compared to placebo for time to all-cause mortality (ACM) or first cardiovascular-related hospitalization (CVH) in both variant (ATTRv) and wild-type (ATTRwt) transthyretin amyloid cardiomyopathy (ATTR-CM) patients from a pre-specified subgroup analysis of ATTRibute-CM, its Phase 3 trial of acoramidis in ATTR-CM. These data were presented at the American College of Cardiology (ACC) Annual Scientific Sessions & Expo in a poster presentation by Margot Davis, M.D. of Vancouver General Hospital, Canada. Acoramidis is a selective small molecule, orally administered, near-complete (≥90%) transthyretin (TTR) stabilizer. “Variant ATTR-CM patients’ condition often presents at an earlier age and progresses more rapidly than patients with wild-type disease, which translates into a worse prognosis in many such patients. We know that pathogenic TTR variant tetramers are less stable than the wild-type tetramer and this property is directly responsible for the more aggressive disease trajectory for these patients. The findings from the ATTRibute-CM trial clearly demonstrate that the rapid and sustained increases in serum TTR levels upon initiation of acoramidis treatment in variant ATTR-CM patients were similar if not greater than those observed in wild-type ATTR-CM patients,” said Jonathan Fox, M.D., Ph.D., president and chief medical officer of BridgeBio Cardiorenal. “This provides evidence that acoramidis is the only disease-modifying therapy that provides near-complete stabilization of TTR, improving clinical outcomes in both variant and wild-type ATTR-CM patients to an extent that is independently statistically significant in both subgroups.” “Given the significant unmet need for patients with ATTRv-CM, we are highly encouraged by the magnitude of efficacy seen with acoramidis. A pre-specified analysis in the ATTRv-CM population has shown a statistically significant 59% hazard reduction for the composite of ACM and CVH at Month 30, demonstrating that acoramidis can make a profound impact on patients’ lives,” said Kevin Alexander, M.D. of Stanford University School of Medicine. Relative increases in serum TTR concentrations resulting from greater TTR stability have been associated with reduced risk of all-cause and cardiovascular mortality in the general population in recent literature.1 The serum TTR level increase with acoramidis was accompanied by a significant reduction in the risk of ACM or first CVH versus placebo in both the ATTRv-CM (59.1% risk reduction, .41 hazard ratio) and ATTRwt-CM (31.2% risk reduction, .69 hazard ratio) subgroups. Acoramidis treatment also led to a greater proportional increase in serum TTR in ATTRv-CM patients and achieved similar absolute serum TTR levels in both ATTRv- and ATTRwt-CM patients, which is an in vivo reflection of acoramidis’ near-complete (≥90%) TTR stabilization. Additional acoramidis poster presentations and moderated posters at the ACC Annual Scientific Sessions & Expo included: Acoramidis Improves NYHA Class at Month 30 Versus Placebo in Patients with ATTR-CM: Results from the ATTRibute-CM Study shared by Kevin Alexander, M.D. of Stanford University School of Medicine In the ATTRibute-CM study, acoramidis treatment resulted in a greater proportion of patients whose New York Heart Association (NYHA) Class was stable or improved at Month 30 vs placebo, indicating better stabilization in their heart failure symptoms and functional status. The NYHA classification system categorizes heart failure patients into four classes based on a clinical assessment of their physical activity limitations and associated symptoms due to their condition In Participants Treated with Acoramidis, Addition of Concomitant Tafamidis Did Not Further Increase Serum TTR Levels shared by Mathew Maurer, M.D. of Columbia University Irving Medical Center The poster showed that in patients with ATTR-CM, treatment with acoramidis significantly increased serum TTR levels whether compared to placebo alone or in those who received placebo as well as tafamidis. Conversely, the addition of tafamidis to acoramidis did not demonstrate any further increase in serum TTR levels, reflecting the lack of any additional stabilization benefit of serum TTR from tafamidis in vivo, as previously demonstrated in vitro. The safety profile in this limited dataset of concomitant acoramidis and tafamidis was similar to the overall safety profile of acoramidis alone Primary Endpoint Efficacy Results in the ATTRibute-CM Study: Pre-specified Sensitivity Analyses Addressed Tafamidis Use shared by Daniel P. Judge, M.D. of Medical University of South Carolina Pre-specified analyses showed consistent results with the primary analysis, demonstrating that the concomitant use of tafamidis did not alter the statistical significance of the primary efficacy endpoint Acoramidis-mediated Early Increase in Serum Transthyretin Level Reduces Cardiovascular-related Hospitalizations and Mortality: Insights from the ATTRibute-CM Study shared by Nitasha Sarswat, M.D. of UChicago Medicine In this post-hoc analysis of ATTRibute-CM, incremental increases in serum TTR levels on Day 28, achieved with acoramidis, may independently predict greater reduction in risks of cardiovascular mortality and of first CVH in patients with ATTR-CM Robustness of Primary Endpoint Efficacy Results with Acoramidis in ATTR-CM in the ATTRibute-CM Study: Pre-specified NT-proBNP Sensitivity Analyses shared by Jan Griffin, M.D. of Medical University of South Carolina This poster shows that pre-specified sensitivity analyses using higher N-terminal pro-type natriuretic peptide (NT-proBNP) thresholds for declaring a difference in the changes in NT-proBNP levels between the treatment arms showed consistent efficacy favoring acoramidis in patients with ATTR-CM. This confirms the robustness of the acoramidis treatment effect regardless of NT-proBNP progression thresholds Geographic Healthcare Disparities and Diagnostic Trends Among Patients with Transthyretin Amyloid Cardiomyopathy shared by Joshua Mitchell, M.D. of Washington University School of Medicine in St. Louis Findings presented show that the diagnosed prevalence of amyloid has significantly increased since 2017 in the setting of available treatment, improved awareness and less invasive diagnostics. However, there remain geographic disparities and racial differences in ATTR-CM prevalence Acoramidis is approved as Attruby by the U.S. FDA and is approved as BEYONTTRA by the European Commission and Japanese Pharmaceuticals and Medical Devices Agency with all labels specifying near-complete stabilization of TTR. More data on the benefit of Attruby for ATTRv-CM patients is planned for future medical meetings. 1Christoffersen M et al. Transthyretin Tetramer Destabilization and Increased Mortality in the General Population. JAMA Cardiol. 2024 Dec 4:e244102. About Attruby™ (acoramidis)Attruby is the first near-complete (≥90%) stabilizer of Transthyretin (TTR) approved in the U.S. for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization. Attruby was generally well-tolerated. The most common side effects were mild and included diarrhea and abdominal pain that were resolved without drug discontinuation. BridgeBio offers an extensive suite of programs to help patients access our medicines. About BridgeBioBridgeBio Pharma (BridgeBio; NASDAQ:BBIO) is a new type of biopharmaceutical company founded to discover, create, test, and deliver transformative medicines to treat patients who suffer from genetic diseases. BridgeBio’s pipeline of development programs ranges from early science to advanced clinical trials. BridgeBio was founded in 2015 and its team of experienced drug discoverers, developers and innovators are committed to applying advances in genetic medicine to help patients as quickly as possible. For more information visit bridgebio.com and follow us on LinkedIn, Twitter and Facebook. BridgeBio Forward-Looking StatementsThis press release contains forward-looking statements. Statements in this press release may include statements that are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended (the Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act), which are usually identified by the use of words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “seeks,” “should,” “continue,” “will,” and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act. These forward-looking statements, including statements relating to the clinical and therapeutic potential of our programs and product candidates, including our clinical development program for acoramidis for patients with transthyretin amyloid cardiomyopathy and the statements regarding the potential clinical benefits or of potential benefits for ATTR-CM patients in the quotes of Dr. Alexander, reflect our current views about our plans, intentions, expectations and strategies, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, and strategies as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations, or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a number of risks, uncertainties and assumptions, including, but not limited to, initial and ongoing data from our preclinical studies and clinical trials not being indicative of final data, the potential size of the target patient populations our product candidates are designed to treat not being as large as anticipated, the design and success of ongoing and planned clinical trials, future regulatory filings, approvals and/or sales, despite having ongoing and future interactions with the FDA and other regulatory agencies to discuss potential paths to registration for our product candidates, the FDA or such other regulatory agencies not agreeing with our regulatory approval strategies, components of our filings, such as clinical trial designs, conduct and methodologies, or the sufficiency of data submitted, the continuing success of our collaborations, our ability to obtain additional funding, including through less dilutive sources of capital than equity financings, potential volatility in our share price, the impacts of current macroeconomic and geopolitical events, including changing conditions from, hostilities in Ukraine, and in Israel and the Gaza Strip, increasing rates of inflation and changing interest rates, on business operations and expectations, as well as those risks set forth in the Risk Factors section of our most recent Annual Report on Form 10-K and our other filings with the U.S. Securities and Exchange Commission. Moreover, we operate in a very competitive and rapidly changing environment in which new risks emerge from time to time. These forward-looking statements are based upon the current expectations and beliefs of our management as of the date of this press release, and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Except as required by applicable law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. BridgeBio Media Contact:Bubba Murarka, EVP Communicationscontact@bridgebio.com (650)-789-8220