ABBOTT PARK, Ill., Aug. 30, 2017 /PRNewswire/ — Abbott (NYSE: ABT) today announced it has initiated a U.S. pivotal clinical study evaluating the safety and effectiveness of a modified version of its AMPLATZER™ device designed to correct a common congenital heart defect that occurs in approximately 80,000 i,ii pre-term infants in the U.S. each year. […]
Coronary/Structural Heart
ESC Congress 2017: BIOTRONIK’s Orsiro Drug-Eluting Stent Outperforms Xience in BIOFLOW-V Trial
Landmark Data Published by The Lancet Demonstrates Statistically Significant Lower Event Rates with Orsiro BARCELONA, Spain, August 28, 2017 – BIOTRONIK today announced data from the BIOFLOW-V randomized trial comparing Orsiro1 and Xience2 drug-eluting stents (DES) with 12-month target lesion failure (TLF) as the primary endpoint proving non-inferiority. Results presented at the […]
Philips and HeartFlow announce global collaboration agreement
Global collaboration aims to accelerate access to tools to determine extent of coronary artery disease and improve patient care Amsterdam, the Netherlands and Redwood City, Calif. – Royal Philips (NYSE: PHG, AEX: PHIA) and HeartFlow, Inc. announced today that they have entered into a collaboration agreement with the goal of […]
Boehringer Ingelheim Release: Pradaxa (Dabigatran Etexilate) Dual Therapy Showed Lower Rates Of Major Bleeding Versus Triple Therapy With Warfarin In Atrial Fibrillation Patients Undergoing Stent Placement
RE-DUAL PCI™ showed large reductions in the incidence of bleeding complications if Pradaxa® dual therapy was used instead of warfarin triple therapy. Both Pradaxa® doses tested in RE-DUAL PCI™ have been approved for stroke prevention in atrial fibrillation. Data were presented as a late-breaker at the ESC Congress 20171 and published in the […]
Bayer (BAY) Release: In Canadian-Led Phase III Clinical Study, Xarelto® When Combined With ASA Significantly Lowered The Combined Risk Of Stroke, Cardiovascular Death, And Heart Attack In Patients With Chronic Coronary Or Peripheral Artery Disease By 24%
Bleeding rates were low, and while major bleeding was increased, notably, there was no significant increase in intracranial or fatal bleeding1. This combination regimen demonstrated a substantial improvement in net clinical benefit of 20%1. Data from Canadian-led COMPASS study, revealed at ESC Congress 2017, included 27,395 patients globally and […]
Androgen Deprivation Therapy Associated with Higher Risk of Heart Failure in Men with Early-Stage Prostate Cancer
PASADENA, Calif., Aug. 24, 2017 /PRNewswire/ — Men with localized prostate cancer who received androgen deprivation therapy, a hormone treatment, were at significantly higher risk of heart failure than men who did not receive this therapy, according to a Kaiser Permanente study published today in the British Journal of Cancer. In the past, […]
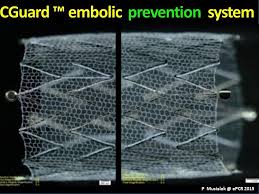
InspireMD Shares CGuard EPS Video Testimonials From European Key Opinion Leaders
TEL AVIV, ISRAEL–(Marketwired – August 22, 2017) – InspireMD, Inc. (NYSE American: NSPR) (NYSE MKT: NSPR) (NYSE American: NSPR.WS) (NYSE MKT: NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced it has posted video testimonials about its CGuard™ Embolic Prevention System from […]
BioVentrix Announces First Patient Enrolled In IDE Study Of The Revivent TC Transcatheter Ventricular Enhancement Treatment For Ischemic Cardiomyopathy
SAN RAMON, Calif. and CAMBRIDGE, England, Aug. 21, 2017 /PRNewswire/ — BioVentrix, Inc. a pioneer of technologies and procedures for less invasive treatment of heart failure (HF), today announced enrollment of the first patient in the international arm of the ALIVE pivotal clinical trial. The trial is designed to demonstrate the safety and effectiveness of […]
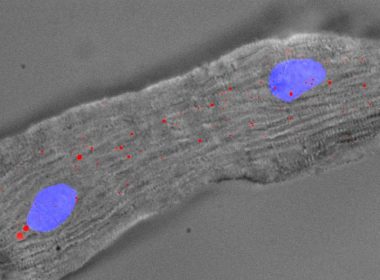
Self Healing Heart cells May Regenerate Repair of Damaged Hearts
Repairing damaged hearts with self-healing heart cells PUBLIC RELEASE: 21-AUG-2017, NATIONAL UNIVERSITY HEALTH SYSTEM Singapore – New research has discovered a potential means to trigger damaged heart cells to self-heal. The discovery could lead to groundbreaking forms of treatment for heart diseases. For the first time, researchers have identified a long non-coding […]
BioStable Science & Engineering Announces FDA Clearance of the HAART™ 200 Aortic Annuloplasty Device for Bicuspid Aortic Valve Repair
AUSTIN, Texas–(BUSINESS WIRE)–BioStable Science & Engineering, Inc. announced today it has received FDA market clearance for the HAART 200 Aortic Annuloplasty Device, the first and only annuloplasty device designed specifically for bicuspid aortic valve repair. With FDA clearance of both the HAART 300 and HAART 200 Aortic Annuloplasty Devices, BioStable […]