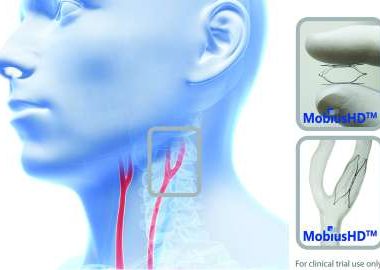
MOUNTAIN VIEW, Calif., Aug. 15, 2017 (GLOBE NEWSWIRE) — Vascular Dynamics, Inc., (VDI) a privately held medical device company developing novel solutions for the treatment of hypertension, today announces that the United States Food and Drug Administration (FDA) has approved the company’s Investigational Device Exemption (IDE) application to initiate its […]
Coronary/Structural Heart
Medtronic Announces Randomized Global Resolute Onyx(TM) DES One-Month Dual Antiplatelet Therapy Study to Address Critical Unanswered Question in Interventional Cardiology
DUBLIN- August 14, 2017 – Medtronic plc (NYSE: MDT) today announced a global randomized clinical trial that will evaluate one-month dual antiplatelet therapy (DAPT) – the combination of aspirin and an anti-clotting medication – in patients implanted with the Resolute Onyx(TM) Drug-Eluting Stent (DES) during percutaneous coronary intervention (PCI). Designed to […]
Abbott Leads Way in First Clinical Trial of Minimally Invasive Clip-Based Repair System for Leaky Tricuspid Heart Valves
ABBOTT PARK, Ill., Aug. 9, 2017 — Abbott today announced that the first patient has been enrolled in a clinical study to evaluate a minimally invasive clip-based repair system for treating people with moderate or severe tricuspid regurgitation (TR), a common condition affecting the right side of the heart. The […]
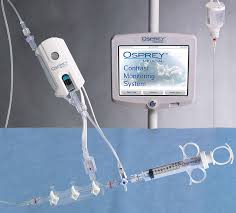
Osprey Medical Improving Cardiac Diagnostics, Raises Capital and Soaring Sales
Osprey Medical (ASX:OSP) based in Minnetonka, MN continues to make strong progress toward their mission of making coronary angiography (heart x-ray imaging) safer for patients with poor kidney function. They are rapidly growing US sales with a direct sales force; in Q2 2017 sales were up 240% over the prior […]
First Cancer, Now Heart Disease…Color Genomics
A New Heart Disease Test Flings Color Genomics Into A Battle About Genetics In Medicine Matthew Herper , Forbes Staff – covering science and medicine. Color Genomics, a Burlingame, Calif.-based startup that has made a name for itself by selling tests for genes that dramatically raise the risk of cancer, is […]
Cardiovascular Systems Releases Data on Peripheral Liberty Trial
This study recaps one year and 1200 patients including use of the Diamond Back 360. Cardiovascular Systems touts 1-year, sub-analysis data from Liberty PVI study AUGUST 10, 2017 BY FINK DENSFORD, MassDevice Cardiovascular Systems (NSDQ:CSII) today released both 1-year and a sub-analysis results from the Liberty 360 clinical trial of peripheral interventions, including those […]
J&J content with it’s Cardio Business…. so far
There has been a ton of consolidation in the med tech sector and one name that is always mentioned is Johnson & Johnson. A lot of speculation has been made about the state of their cardiology business, especially after cutting ties with their old Cordis division. Analyst: Johnson & Johnson […]
Philips completes acquisition of The Spectranetics Corporation
Amsterdam, the Netherlands and Colorado Springs, CO, U.S. – Royal Philips (NYSE: PHG; AEX: PHIA), a global leader in health technology, today announced that it has completed the acquisition of The Spectranetics Corporation (NASDAQ: SPNC), a U.S.-based global leader in vascular intervention and lead management solutions. Spectranetics’ financial results will be consolidated as part of […]
John K. Bakewell Joins Corindus Vascular Robotics Board of Directors
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE American: CVRS], a leading developer of precision vascular robotics, today announced that John K. Bakewell has been appointed to its board of directors, effective immediately. Mr. Bakewell will serve as the Chair of the Audit Committee. President and Chief Executive Officer Mark Toland stated, […]
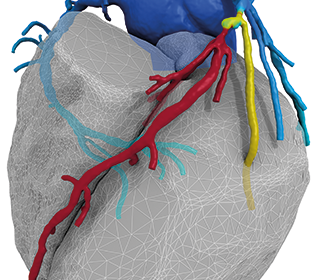
HeartFlow Announces Four Blue Cross Blue Shield Companies Issue Positive Medical Policies on Non-Invasive HeartFlow® FFRct Analysis for Coronary Artery Disease
REDWOOD CITY, Calif.–(BUSINESS WIRE)–HeartFlow, Inc. today announced that Horizon Blue Cross Blue Shield of New Jersey (Horizon BCBSNJ), Blue Cross Blue Shield of Arizona, Blue Cross of Idaho and Blue Cross Blue Shield of Kansas City have each issued a positive medical policy for the HeartFlow® FFRct Analysis, a first-of-its-kind noninvasive technology […]