BOULDER, Colo.–(BUSINESS WIRE)–Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the Company will present data on EDG-7500 at the American College of Cardiology’s Annual Scientific Session (ACC.24). EDG-7500 is a first-in-class oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM and other diseases of diastolic dysfunc
Coronary/Structural Heart
Polymedco announces US FDA clearance of the PATHFAST high-sensitivity cardiac troponin I (hs-cTnI-II) test as an aid in the diagnosis of myocardial infarction
PATHFAST hs-cTnI-II becomes first and only hs-cTn test cleared for point-of-care use in the United States, delivering results up to three times faster than core lab testing CORTLANDT MANOR, N.Y., March 27, 2024 /PRNewswire/ — The PATHFAST hs-cTnI-II, a breakthrough high-sensitivity…
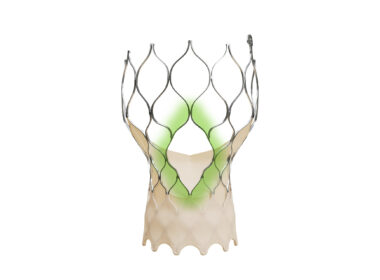
Medtronic announces FDA approval of newest-generation Evolut TAVR system for treatment of symptomatic severe aortic stenosis
The Evolut™ FX+ TAVR system leverages market-leading valve performance with addition of larger windows to facilitate coronary access DUBLIN, March 27, 2024 /PRNewswire/ — Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced that the United States Food and…
HeartFlow Initiates DECIDE Registry to Evaluate Utility of HeartFlow AI-Enabled Plaque Analysis for Patients with Suspected Coronary Artery Disease
The largest prospective registry of its kind will build upon clinical data supporting the use of plaque insights to empower clinical decision making for patients with suspected coronary artery disease The largest prospective registry of its kind will build upon clinical data supporting the use of plaque insights to empower clinical decision making for patients with suspected coronary artery disease
Cleerly® ISCHEMIA™ Demonstrates Robust Diagnostic Accuracy and Prognostic Utility in Analyses from Large Scale Clinical Trials
DENVER–(BUSINESS WIRE)–Cleerly, the company on a mission to create a new standard of care to aid in the diagnosis of heart disease, shared findings from a study published online in the Journal of the American College of Cardiology: Cardiovascular Imaging on March 13, 2024. The study describes the validation of Cleerly’s artificial intelligence-guided quantitative coronary CT angiography (AI-QCT) ISCHEMIA technology for diagnostic accuracy and prognostic risk stratification. In two trials,1-2
Bristol Myers Squibb to Present Data at the American College of Cardiology Annual Scientific Session 2024 Reinforcing Extensive Body of Evidence in Clinical and Real-World Settings Across Cardiovascular Portfolio
Analysis from a 10-month post-launch evaluation of the REMS Program finds 2.8% incidence of LVEF <50% in over 1500 patients, strengthening the safety profile of CAMZYOS® (mavacamten) for NYHA class II-III obstructive hypertrophic cardiomyopathy Real-world data reaffirm therapeutic value and treatment benefit of CAMZYOS in improving cardiac symptoms and NYHA class in patients with obstructive […]
RWJBarnabas Health and Rutgers Health to Showcase Breakthrough Cardiology Data at the American College of Cardiology Scientific Session & Expo
59 abstract presentations will analyze treatments and programs revolutionizing cardiovascular care WEST ORANGE, N.J., March 25, 2024 /PRNewswire/ — Physician-scientists from RWJBarnabas Health and Rutgers Health will present an extensive portfolio of innovative cardiovascular data from…
U.S. FDA Approves Broad New Labels for Esperion’s NEXLETOL® and NEXLIZET® to Prevent Heart Attacks and Cardiovascular Procedures in Both Primary and Secondary Prevention Patients, Regardless of Statin Use
ANN ARBOR, Mich.–(BUSINESS WIRE)–Esperion (NASDAQ: ESPR) today announced that the United States Food and Drug Administration (FDA) has approved broad new label expansions for NEXLETOL® (bempedoic acid) Tablets and NEXLIZET® (bempedoic acid and ezetimibe) Tablets based on positive CLEAR Outcomes data that include indications for cardiovascular risk reduction and expanded LDL-C lowering in both primary and secondary prevention patients. In addition, the enhanced labels support the use of NEXLET
JenaValve’s Trilogy THV System Highlighted at CRT 2024
IRVINE, Calif., March 19, 2024 (GLOBE NEWSWIRE) — JenaValve Technology, Inc., developer and manufacturer of the Trilogy™ Transcatheter Heart Valve (THV) System, today announced highlights from the Cardiovascular Research Technologies (CRT) Conference 2024. JenaValve’s Trilogy THV system, a TAVR system designed to treat patients with symptomatic, severe aortic regurgitation (ssAR), and symptomatic, severe aortic stenosis (ssAS), was featured in multiple scientific sessions highlighting the significant unmet need for devices to treat ssAR.
Cerus Corporation Announces Positive Topline Results for the Phase 3 Clinical Trial of the INTERCEPT Blood System for Red Blood Cells in Cardiovascular Surgery Patients
CONCORD, Calif.–(BUSINESS WIRE)–Cerus Corporation (Nasdaq: CERS) today announced positive topline results for ReCePI, a pivotal Phase 3 clinical trial of pathogen reduced INTERCEPT Red Blood Cells (INTERCEPT RBCs) transfused to complex cardiac surgery patients. The trial met its primary efficacy endpoint, demonstrating non-inferiority for INTERCEPT RBCs compared to conventional RBCs as measured by the incidence of acute kidney injury (AKI) following transfusion of study RBCs. AKI is a sensiti