New clinical data show AI-driven gains in aneurysm growth detection, stroke interpretation accuracy, and radiology workflow efficiency across enterprise imaging environments. SAN MATEO, CA (February 4, 2026) – RapidAI, the pioneer of deep clinical AI and global leader in enterprise imaging, today announced details of 28 scientific abstracts accepted at the International Stroke Conference (ISC) […]
Neuro
Vesalio Expands International Neurovascular Portfolio with CE Mark of NeVa VS and NeVa 3.0 mm and Receives Additional FDA 510(k) Clearance for its Aspiration Catheters
Company advances global neuro-intervention footprint with new CE-marked devices for vasospasm and stroke, and expanded U.S. indications for aspiration catheters. PLANO, Texas, Feb. 10, 2026 /PRNewswire/ — Vesalio, a global leader in vascular intervention, today announced CE Mark…
Balt’s Squid™ Liquid Embolic Receives FDA Approval for Adjunctive Treatment for Patients with Chronic Subdural Hematomas
BOSTON, Feb. 04, 2026 (GLOBE NEWSWIRE) — Balt, Inc., a global technology leader in neurovascular devices, today announced the Premarket Approval (PMA) of the Squid™ liquid embolic agent. Squid is approved for the embolization of the middle meningeal artery (MMA) as an adjunct to usual care treatment in patients with large symptomatic chronic subdural hematoma(s) (cSDH).
Brainomix Launches Award-Winning Next Generation Stroke AI Platform with Novel Net Water Uptake Technology at the International Stroke Conference (ISC)
Brainomix 360 Stroke Next Generation has been awarded the prestigious Red Dot Design Award for excellence in usability and innovation An industry-first net water uptake feature unlocks deeper insights into stroke injury that can better inform treatment decisions New research will be…
Imperative Care Launches Zoom 4S Catheter to Further Advance Company’s Zoom Stroke System for Ischemic Stroke Treatment
A Pivotal Step in Optimizing the ADAPT 2.0 Technique for Speed and Simplicity with a Single System Setup ADAPT 2.0 with the Zoom Stroke System CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced the commercial launch and first patient cases of the new Zoom 4S Catheter – an advanced aspiration thrombectomy […]
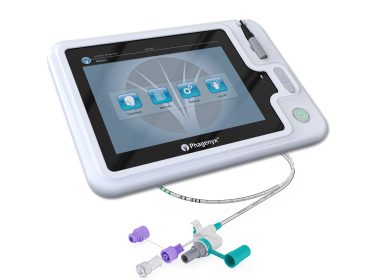
Pharyngeal Electrical Stimulation Recognized as Important Tool for Post-Stroke Recovery in New American Heart Association and American Stroke Association Guideline
Inclusion in new Acute Ischemic Stroke guidelines underscores clinical utility of Phagenyx for accelerating recovery from swallowing difficulties (dysphagia) in stroke patients NASHVILLE, Tenn., Jan. 27, 2026 /PRNewswire/ — Phagenesis, a commercial-stage company with a first-of-its-kind…
Kaneka Medical America LLC Announces U.S. Launch of WaveSelect 1014 Guidewire
Exclusive U.S. Distribution Partnership with Enlight Medical Ltd. Enables Kaneka Medical America LLC to Bring Novel Neurovascular Guidewire Technology to the U.S. Market Exclusive U.S. Distribution Partnership with Enlight Medical Ltd. Enables Kaneka Medical America LLC to Bring Novel Neurovascular Guidewire Technology to the U.S. Market
Ceribell Receives FDA Breakthrough Device Designation for LVO Stroke Detection and Monitoring Solution
Clearance would position the Ceribell System as the first and only point-of-care electroencephalography (EEG) technology to aid in detection and monitoring of Large Vessel Occlusion (LVO) stroke in the hospital setting Clearance would position the Ceribell System as the first and only point-of-care electroencephalography (EEG) technology to aid in detection and monitoring of Large Vessel Occlusion (LVO) stroke in the hospital setting
Hyperfine Announces FDA Clearance of the First Optive AI™ Software Update with Advanced Diffusion Imaging Capability, Focused on Enhancing Stroke Detection with the Swoop® System
A new multi-direction DWI sequence, the latest Swoop® system software, and the first advancement in Hyperfine’s Optive AI™ software, delivers clearer, higher-quality images for stroke diagnosis, enhancing the value of the Swoop® system in acute neurological care. GUILFORD, Conn.–(BUSINESS WIRE)–Hyperfine, Inc. (Nasdaq: HYPR), the groundbreaking health technology company that has redefined […]
Imperative Care Presents Positive Real-World Data from Ischemic Stroke Patients Treated with ADAPT 2.0 Using the Zoom Stroke System
Late-Breaking Data Presented at Society of Vascular and Interventional Neurology Annual Meeting Demonstrated Excellent Reperfusion and Rapid Procedure Times with the Zoom Stroke System Using Large-Bore Intracranial Access, Asymmetric Aspiration, and Continuous Dual Aspiration CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced the presentation of late-breaking, real-world data from a multi-center review […]