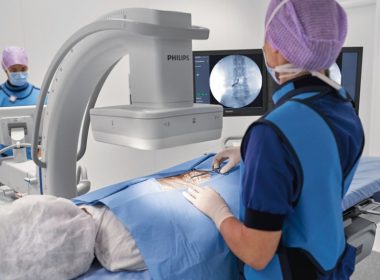
AMSTERDAM, Feb. 18, 2019 /PRNewswire/ — Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Philips Zenition, its new mobile C-arm imaging platform. Mobile C-arms are X-ray systems that are brought into the operating room (OR) to provide live image guidance during a wide range of surgeries including […]
Peripheral/Endo
Affluent Medical Releases Impressive Results from Scope 1 Clinical Trial Validating Efficacy of KARDIOZIS Technology
PARIS–(BUSINESS WIRE)–Affluent Medical (Paris:ALAM), a French medtech company specialized in innovative, minimally invasive implants designed to restore key physiological functions for patients suffering from heart and vascular diseases, as well as urinary incontinence, today announces positive results from its SCOPE 1 clinical trial validating the efficacy of KARDIOZIS technology. Results have been […]
Medtronic Receives FDA Approval on Expanded Indication for Pipeline Flex Embolization Device
DUBLIN – February 7, 2019 – Medtronic plc (NYSE:MDT) announced today that it has received U.S. Food and Drug Administration (FDA) approval on an expanded indication for its Pipeline(TM) Flex embolization device.1 Previously indicated for the endovascular treatment of adults with large or giant wide-necked intracranial aneurysms (IAs) in the internal carotid […]
VentureMed Group Names J. Robert Paulson Jr. Chief Executive Officer
Toledo, Ohio, February 5, 2019 – VentureMed Group, Inc., a privately-held medical device company that develops and markets the FLEX Vessel Prep™ System to treat peripheral arterial disease (PAD) and stenoses of arteriovenous (AV) fistulas and grafts, announced today that J. Robert Paulson, Jr., has been appointed president and chief […]
According to THE SAGE GROUP, Thrombus is Highly Prevalent in Both Symptomatic and Asymptomatic Peripheral Artery Disease (PAD) Patients
BEAUFORT, S.C.–(BUSINESS WIRE)–A new analysis published by THE SAGE GROUP concludes that occlusive thrombus is present in 3.6 million PAD patients. “Occlusive thrombus in the leg arteries has serious consequences including ischemic symptoms, and in severe cases amputation. Furthermore, its presence adversely affects interventional procedure outcomes and costs. Thrombus interferes […]
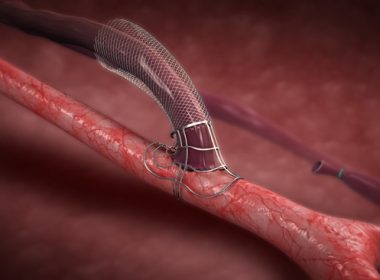
Teleflex Announces U.S. FDA Premarket Approval of MANTA™ Vascular Closure Device
WAYNE, Pa.–(BUSINESS WIRE)–Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies for critical care and surgery, has announced that it received premarket approval (PMA) from the U.S. Food and Drug Administration for the MANTA™ Vascular Closure Device – the first commercially available biomechanical vascular closure device designed specifically […]
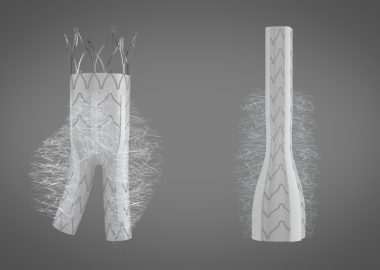
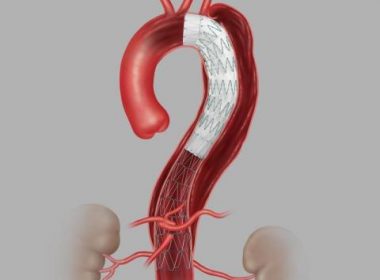
Cook Medical Receives U.S. FDA Approval for Aortic Dissection Device
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Today, Cook Medical announced its recent approval from the U.S. FDA for its Zenith Dissection Endovascular System. The system, consisting of a proximal stent-graft component and a distal bare stent component, provides physicians a less invasive alternative to open surgery for repair of Type B dissections of the […]
CHMP Recommends Approval of Praluent® (alirocumab) Injection to Reduce Cardiovascular Risk in People with Established Atherosclerotic Cardiovascular Disease
TARRYTOWN, N.Y. and PARIS, Feb. 4, 2019 /PRNewswire/ — Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) and Sanofi today announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion for Praluent® (alirocumab) Injection, recommending a new indication to reduce cardiovascular risk by lowering low-density lipoprotein cholesterol (LDL-C) levels as an […]
Israeli Start-up Laminate, Developer of a Vascular Support Solution for Dialysis Patients, Has Completed a Capital Raising Round of $12 Million
TEL-AVIV, Israel, Jan. 31, 2019 /PRNewswire/ — The Israeli start-up Laminate Medical Technologies, developer of a vascular support device that is implanted in patients requiring dialysis, announces a capital raising round of $12 million. This round brings total investment in the company to date to $24 million. Participating in the current round is the leading dialyzer […]
XableCath Crossing Catheters FDA Cleared
SALT LAKE CITY, Jan. 30, 2019 (GLOBE NEWSWIRE) — XableCath, Inc., announced that its XableCath™ blunt and abrasion tip catheters, were cleared by the FDA as Peripheral Crossing Catheters. The catheters have been safely and successfully used to cross challenging lesions in both arterial CTO’s and chronic obstructive venous lesions. […]