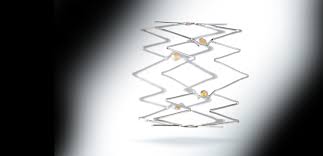
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the first commercial use of its Tack Endovascular System® in multiple hospitals within Germany. A novel therapy for dissection repair following balloon angioplasty, the Tack® implant is a first-of-its kind device for patients with peripheral […]
Peripheral/Endo
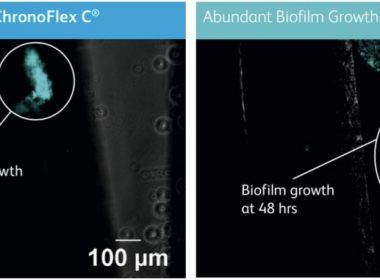
Access Scientific announces the first non-coated intravascular catheter material–ChronoFlex®C with BioGUARD™–proven in vitro to inhibit bacterial attachment and biofilm formation
SAN DIEGO, Jan. 7, 2019 /PRNewswire/ — Access Scientific, LLC today announced publication, in the journal Medical Devices: Evidence & Research, of a groundbreaking study proving the superiority of POWERWAND™ catheter material, Chronoflex®C with BioGUARD™ (as compared with the standard polyurethane) with respect to bacterial attachment and biofilm formation. Utilizing state-of-the-art fluorescent microscopy, the […]
Endologix Takes Decisive Action to Optimize Patient Outcomes by Ensuring Nellix System Used Only within Current Indications
IRVINE, Calif.–(BUSINESS WIRE)–Endologix® Inc. (Nasdaq: ELGX) (“Endologix” or the “Company”), a developer and marketer of innovative treatments for aortic disorders, announced today that in order to ensure optimal outcomes for patients, unrestricted sales and use of the Nellix System will cease immediately, and the product will only be available for […]
Hemostemix Announces Two Additional Sites Enrolled in Phase II Clinical Trial
CALGARY, Alberta, Jan. 03, 2019 (GLOBE NEWSWIRE) — Hemostemix Inc. (“Hemostemix” or the “Company”) (TSX VENTURE: HEM; OTCQB:HMTXF) is pleased to announce that it has received all approvals from two new medical centers to be clinical trial sites for the Company’s Phase II clinical trial for critical limb ischemia (“CLI”). […]
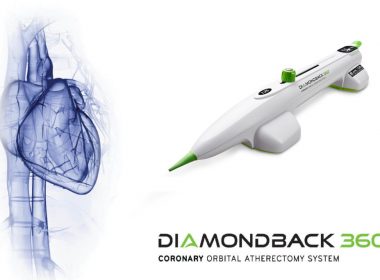
Cardiovascular Systems, Inc. Receives Approval for the Diamondback 360® Coronary Orbital Atherectomy System with Classic Crown and New ViperWire Advance® FlexTip Guidewire in Japan
ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, announced today that Japan’s Ministry of Health, Labor and Welfare (MHLW) has approved the Diamondback 360®Coronary Orbital Atherectomy System (OAS) with Classic Crown […]
Intact Vascular Announces Enrollment Completion of TOBA II BTK Clinical Trial
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced its Tack Optimized Balloon Angioplasty II BTK (TOBA II BTK) clinical trial successfully completed enrollment, ahead of schedule. This study further augments Intact Vascular’s robust clinical program and is notably the first pivotal trial investigating a […]
InspireMD Announces Publication of Meta-Analysis Citing Benefits of Next Generation Mesh-Covered Carotid Stent Systems
TEL AVIV, Israel, Dec. 18, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE American: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of carotid artery disease, today announced the publication of a meta-analysis of four clinical studies involving dual layered and […]
Hancock Jaffe Receives Approval for First-in-Human VenoValve Study
IRVINE, Calif., Dec. 17, 2018 (GLOBE NEWSWIRE) — Hancock Jaffe Laboratories, Inc. (Nasdaq: HJLI, HJLIW), a company specializing in medical devices that restore cardiac and vascular health, announced today that it has received regulatory approval from INVIMA, the Colombian equivalent of the U.S. Food and Drug Administration, for its first-in-human […]
Aortica Corp. Announces FDA Approves Medtronic Valiant NAVION™ for Inclusion in Starnes’ Physician-Sponsored IDE
BELLEVUE, Wash.–(BUSINESS WIRE)–Aortica Corporation today announced that FDA has approved a supplement to an ongoing Physician Sponsored IDE study at the University of Washington (UW) sponsored by Principal Investigator & Chief of Vascular Surgery Dr. Benjamin Starnes. The positive decision allows the use of Medtronic’s Valiant NAVION™ stent graft system […]
Endologix Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4)
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq: ELGX) (the “Company”), a developer and marketer of innovative treatments for aortic disorders, announced today the grant of inducement equity awards to three newly hired employees (together, the “Awardees”). The awards were approved by the Company’s Compensation Committee, which is comprised of independent Directors, on December 5, […]