MINNEAPOLIS, April 23, 2025 /PRNewswire/ — VentureMed Group, Inc., a privately held leader in medical device innovations for arteriovenous (AV) access and peripheral arterial disease (PAD), announced data presented at the Charing Cross Symposium, April 23 – 25th, London, England….
Peripheral/Endo
Surmodics Announces Publication of TRANSCEND Trial, Highlighting Drug-Delivery Technology of its SurVeil™ Drug-Coated Balloon
European Journal of Vascular and Endovascular Surgery Publishes Results Showing Comparable Safety and Efficacy of SurVeil™ DCB Despite IN.PACT™ Admiral™ DCB having 75% Higher Paclitaxel Dose. Figure 1. SurVeil™ DCB balloon coating (above) vs IN.PACT™ Admiral™ DCB balloon coating (below). EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (Nasdaq: SRDX), a leading provider […]
R3 Vascular Announces First Patient Treated in ELITE-BTK Pivotal Trial for Below-the-Knee PAD Using MAGNITUDE® Drug Eluting Bioresorbable Scaffold
MOUNTAIN VIEW, Calif., April 22, 2025 (GLOBE NEWSWIRE) — R3 Vascular Inc., a medical device company dedicated to developing and providing novel, best-in-class bioresorbable scaffolds for treating peripheral arterial disease (PAD), is pleased to announce that the first patient in its ELITE-BTK pivotal trial was treated by Dr. Brian DeRubertis, FACS, Chief of the Division of Vascular & Endovascular Surgery at New York-Presbyterian and Weill Cornell Medicine in New York City. The trial evaluates R3 Vascular’s next generation drug eluting bioresorbable scaffold, MAGNITUDE®, for below-the-knee (BTK) PAD, which according to the American Heart Association affects more than 200 million people globally, and 10 to 12 million in the U.S. older than the age of 40. The most common type of PAD affects the lower-extremity where blood flow is reduced to the legs and feet. Each year, approximately 150,000 leg amputations are performed in the U.S. alone.
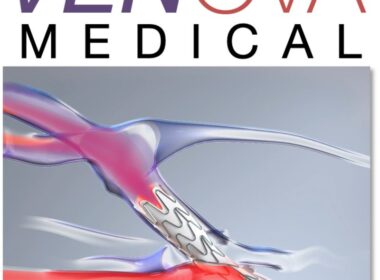
Venova Medical Announces Completion of Enrollment for VENOS-2 IDE Study of the VelocityTM Percutaneous AVF System
Los Gatos, California – Venova Medical, a privately held company developing a next generation technology for the creation of percutaneous arteriovenous fistulas (pAVF) for hemodialysis access, today announced the successful completion of patient enrollment for the company’s VENOS-2 early feasibility study (NCT06712251). This multi-center study is being performed under an Investigational […]
SonoVascular wins Angel Capital Association 2025 Luis Villalobos Award
CHAPEL HILL, N.C., April 16, 2025 /PRNewswire/ — SonoVascular, Inc., a clinical stage medical device company focused on bringing to market its SonoThrombectomy™ System, a safer and more effective treatment of venous thromboembolism (VTE), was awarded the Angel Capital Association’s 2025…
FluidForm Bio™ Advances Vascularized Tissue Engineering with New FRESH™ 3D Bioprinting Breakthrough
WALTHAM, Mass., April 15, 2025 (GLOBE NEWSWIRE) — FluidForm Bio™, a leader in cell therapies for chronic diseases such as type 1 diabetes, today announced a major advancement in vascularized tissue engineering. A newly published study in ACS Biomaterials Science & Engineering demonstrates how FRESH™ 3D bioprinting, combined with sacrificial gelatin microparticles, significantly enhances cell viability in thick tissue constructs by improving nutrient diffusion and enabling scalable perfusion strategies.
Cagent Vascular Initiates Patient Enrollment in the Serranator® vs. POBA OCT Study at Columbia University Medical Center
Study Seeks to Confirm Benefits of Cagent’s Unique Serration Balloon Angioplasty Technology vs. Plain Old Balloon Angioplasty (POBA) in treating Peripheral Artery Disease (PAD) in Below-the-Knee Arteries. WAYNE, Pa.–(BUSINESS WIRE)–Cagent Vascular, Inc., the exclusive developer of serration technology for vessel dilation in endovascular interventions, announced its 1st patient enrollment of the […]
Microbot Medical® Shares Results from Its Pivotal Clinical Trial, Achieving 100% Robotic Navigation Success for the LIBERTY® Endovascular Robotic System
Successful robotic navigation was achieved in every case and met the primary endpoint of the study
InspireMD to Present at Upcoming 24th Annual Needham Virtual Healthcare Conference
MIAMI, March 26, 2025 (GLOBE NEWSWIRE) — InspireMD, Inc. (Nasdaq: NSPR), developer of the CGuard™ Prime carotid stent system for the treatment of carotid artery disease and prevention of stroke, today announced management will present at the 24th Annual Needham Virtual Healthcare Conference on Wednesday, April 9, 2025, at 8:00AM Eastern Time / 5:00AM Pacific Time.
Evident Vascular Raises Series B Financing to Advance AI-Powered Intravascular Ultrasound Platform Technology
Funding to accelerate development of next-generation intravascular ultrasound platform and support FDA clearance for U.S. market launch SAN JOSE, Calif.–(BUSINESS WIRE)–Evident Vascular, Inc., a medical technology company developing a best-in-class intravascular ultrasound (IVUS) platform powered by artificial intelligence, today announced the successful closing of its Series B financing with new […]