TEL AVIV, Israel, Oct. 18, 2018 /PRNewswire/ — Goldman Sachs is recognizing Dr. Eyal Orion, founder and CEO of Vascular Graft Solutions as one of the 100 Most Intriguing Entrepreneurs of 2018 at its Builders + Innovators Summit in Santa Barbara, California. Goldman Sachs selected Dr. Orion as one of 100 entrepreneurs from multiple industries to […]
Peripheral/Endo
MC3 Cardiopulmonary Announces FDA Clearance of Crescent™ for Veno-Venous Extracorporeal Membrane Oxygenation (ECMO)
DEXTER, Mich., Oct. 18, 2018 /PRNewswire/ — MC3 (mc3corp.com) today announced the launch of the Crescent™Jugular Dual Lumen Catheter, the first such device cleared by FDA for ECMO (Extracorporeal membrane oxygenation) in the United States. MC3’s Crescent catheter is placed through the jugular vein and is connected to an ECMO system, which removes […]
Bluegrass Vascular Announces Enrollment Completion Of International Post-Market SAVE Registry With Early Positive Results
SAN ANTONIO, Oct. 15, 2018 /PRNewswire/ — Bluegrass Vascular Technologies (Bluegrass Vascular), a private medical technology company focused on innovating lifesaving devices and methods for vascular access procedures, announced today it has successfully completed enrollment of its prospective, multicenter, post-CE Mark, international SAVE (Surfacer® System to Facilitate Access in VEnous Occlusions) Registry. In addition, the company […]
Veryan Medical’s BioMimics 3D receives PMA approval
04 October 2018: Veryan Medical Ltd (Horsham, UK) today announced that the Company has received Premarket Approval (PMA) for the BioMimics 3D Vascular Stent System from the U.S. Food & Drug Administration (FDA). The device is approved for the treatment of symptomatic de novo or restenotic lesions in the native […]
Concept Medical Inc. Raises $60mn for Investigational Device Exemption (IDE) for the World’s First Sirolimus-Coated Balloon
SURAT, India, Oct. 9, 2018 /PRNewswire/ — Concept Medical Inc. Raises $60mn For Investigational Device Exemption (IDE) For The World’s First Sirolimus-Coated Balloon. Concept Medical Inc. (CMI) has approached the FDA for an Investigational Device Exemption (IDE) for their Sirolimus-Coated Balloon (DCB). To support this process, they have raised $60mn (for an undisclosed valuation) from cardiologist and […]
Hemostemix Announces Three Additional Sites Enrolled in Phase II Clinical Trial
CALGARY, Alberta, Oct. 09, 2018 (GLOBE NEWSWIRE) — Hemostemix Inc. (“Hemostemix” or the “Company”) (TSX VENTURE: HEM) is pleased to announce that it has received all approvals from three new medical centers to be clinical trial sites for the Company’s Phase II clinical trial for critical limb ischemia (“CLI”). In […]
Hancock Jaffe Enters into Sponsored Research Agreement for CoreoGraft with World-Renowned Texas Heart Institute
IRVINE, Calif., Oct. 09, 2018 (GLOBE NEWSWIRE) — Hancock Jaffe Laboratories, Inc. (Nasdaq: HJLI, HJLIW), a company specializing in medical devices that restore cardiac and vascular health, announced today that it has entered into a Sponsored Research Agreement with Texas Heart Institute for the development of HJLI’s CoreoGraft®, an off […]
Eximo Medical Receives FDA Clearance for B-Laser™ Atherectomy System to Treat Peripheral Artery Disease (Including ISR)
REHOVOT, Israel–(BUSINESS WIRE)–Eximo Medical Ltd. announced today it has received 510(k) clearance from the U. S. Food & Drug Administration (FDA) for its B-Laser™ Atherectomy System for Peripheral Artery Disease (PAD). B-Laser™ is a transformative 355nm wavelength laser technology designed to address unmet clinical needs for treating multiple vascular indications. The specific indication cleared by the FDA is: […]
Intact Vascular Announces Presentation of TOBA II Pivotal Data
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that data from its Tack Optimized Balloon Angioplasty (TOBA) II pivotal clinical trial will be presented at the 15th Annual VIVA Conference in Las Vegas, NV on November 5-8. The TOBA II trial […]
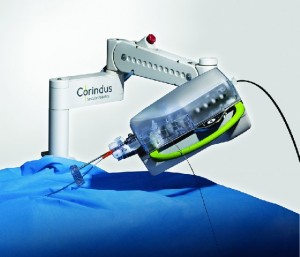
Corindus Technology Successfully used in Multiple Live Complex Robotic-Assisted Coronary Interventions at Transcatheter Cardiovascular Therapeutics 2018
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. (NYSE American: CVRS), a leading developer of precision vascular robotics, announced today that its CorPath GRX System was used for multiple complex robotic-assisted percutaneous coronary interventions (PCI) at the recent Transcatheter Cardiovascular Therapeutics (TCT) 2018 Conference. In the first case at University of Washington […]