HINGHAM, Mass., June 18, 2018 (GLOBE NEWSWIRE) — Microbot Medical Inc. (Nasdaq CM:MBOT), a medical device company specializing in the design and development of transformational micro-robotic medical technologies, today announced that the European Patent Office (EPO) has notified the Company that it will grant a patent for Application No. 12837337.0, […]
Peripheral/Endo
Essential Medical Announces IDE Study Results to be Presented in Late Breaking Clinical Science Session at TVT 2018
MALVERN, Pennsylvania, June 18, 2018 /PRNewswire/ — Essential Medical, Inc., a private medical device company focused on innovative solutions for large bore vascular closure, today announced that the results from the SAFE MANTA IDE clinical trial will be presented at the Transcatheter Valve Therapies (TVT) 2018 congress in Chicago June 20-22. Essential Medical’s MANTA™ system is the […]
Avinger Announces First Patients Enrolled in Post-Market Study Comparing Pantheris OCT Imaging to Intravascular Ultrasound
REDWOOD CITY, Calif., June 14, 2018 (GLOBE NEWSWIRE) — Avinger, Inc. (Nasdaq:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced the initiation of the SCAN study, a post-market study comparing optical coherence tomography (OCT) with intravascular ultrasound (IVUS) as a diagnostic imaging tool in the […]
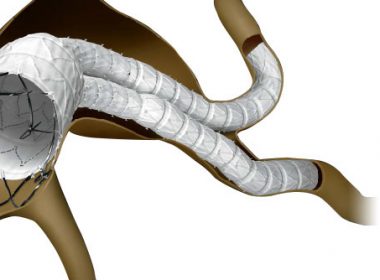
FDA Advisory Committee Votes in Favor of Cardinal Health’s INCRAFT® AAA Stent Graft System for the Endovascular Treatment of Infrarenal Abdominal Aortic Aneurysms
DUBLIN, Ohio, June 12, 2018 /PRNewswire/ — Cardinal Health (NYSE: CAH) today announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee has provided a favorable recommendation on the premarket approval application for INCRAFT® AAA Stent Graft System (INCRAFT). The panel voted 11 to 4 in […]
CryoLife Announces Peer Review Publication of On-X® Aortic Heart Valve PROACT Study
ATLANTA, June 11, 2018 /PRNewswire/ — CryoLife, Inc. (NYSE: CRY), a leading cardiac and vascular surgery company focused on aortic disease, today announces the publication of our clinical study entitled “Anticoagulation and Antiplatelet Strategies After On-X Mechanical Aortic Valve Replacement” in a peer-reviewed medical journal, the Journal of the American College of Cardiology. The publication reports outcomes from […]
InspireMD’s CGuard™ MicroNet™ EPS Featured in a Live Case Transmission to the 2nd DGA Interventional Congress
Tel Aviv, Israel, June 11, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE AMER:NSPR), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced that its CGuard™ MicroNet™ covered Embolic Prevention System (EPS) was successfully featured in a live case transmission on June 8th to […]
Fresenius Medical Care and Humacyte announce strategic global partnership supported by a $150M equity investment
BOSTON, June 11, 2018 /PRNewswire/ — Fresenius Medical Care, the world’s largest provider of dialysis products and services, and Humacyte, Inc., a medical research, discovery and development company, today announced a strategic, global partnership and a $150M USD equity investment. This agreement has the potential to make Humacyte’s investigational human acellular vessel, HUMACYL®, available […]
Micro Medical Solutions Marks First U.S. Implants of MicroStent for Peripheral Artery Disease
WILMINGTON, Mass.–(BUSINESS WIRE)–Micro Medical Solutions (MMS), an innovator in the field of microvascular interventions that aim to improve clinical outcomes and quality of life, announced a major milestone today in the development of its MicroStent technology, a vascular stent specifically designed to reduce below-the-knee amputations caused by critical limb ischemia […]
Biomerics Acquires FutureMatrix Interventional
SALT LAKE CITY, June 4, 2018 /PRNewswire/ — Biomerics LLC, a medical device contract manufacturer headquartered in Salt Lake City, UT is pleased to announce the acquisition of FutureMatrix Interventional, Inc. FMI, located in Athens, TX specializes in the design and manufacture of interventional catheters for the cardiovascular and urinary markets. “We are pleased to add […]
Avinger Announces Presentations and Posters Featuring Lumivascular Technology at New Cardiovascular Horizons Annual Conference
REDWOOD CITY, Calif., May 30, 2018 (GLOBE NEWSWIRE) — Avinger, Inc. (Nasdaq:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced a schedule of sessions and posters highlighting the company’s Lumivascular technology at the New Cardiovascular Horizons (NCVH) annual conference taking place this week in New […]