Results show that first-of-its-kind device has a sustained treatment effect and a positive impact on quality of life Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced positive two-year results from the Tack Optimized Balloon Angioplasty (TOBA) II below-the-knee (BTK) clinical trial. The data show the Philips […]
Peripheral/Endo
SoundBite Medical’s Shock Wave Technology Continues to Crack Through Severe Calcium with the Addition of its First US Site
MONTREAL–(BUSINESS WIRE)–SoundBite Medical Solutions Inc. (SBMS), a medical device company dedicated to developing solutions for the interventional treatment of calcific peripheral and coronary arterial diseases, today announced the use of its novel Active Wire 0.014” platform at a first site in the United States in the successful treatment of patients suffering from […]
AI triage solution for deadly vascular conditions receives FDA clearance and CE Mark
Avicenna.AI announces the introduction of CINA CHEST, including AI tools for detection and emergency triage of pulmonary embolism and aortic dissection MARSEILLE, France , June 2, 2021 /PRNewswire/ — Medical imaging AI specialist Avicenna.AI today announced that it has received certification in the United States and European Union for CINA CHEST, its new AI solution that leverages […]
Cardiovascular Systems, Inc. Announces U.S. Commercial Launch of JADE Over-the-Wire Non-Compliant Peripheral Balloon Catheters
ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, announced today that the full line of OrbusNeich® JADE® percutaneous transluminal angioplasty (PTA) over-the-wire (OTW) balloon catheters is now available in the U.S. CSI […]
SIRONA – The world’s first and largest RCT comparing Sirolimus V/S Paclitaxel balloon for the treatment of Peripheral Arterial Disease progresses rapidly
JENA, Germany, May 24, 2021 /PRNewswire/ — Concept Medical Inc., focused on vascular intervention drug delivery devices, releases status updates of SIRONA Randomized Control Trial (RCT) which compares head-to-head, SIROlimus versus Paclitaxel Drug-Eluting BallooN Angioplasty in femoropopliteal arterial diseases (SIRONA). SIRONA is the world’s first RCT investigating the use of Sirolimus drug coated balloon (DCB) (MagicTouch PTA – Concept Medical) […]
New Study Demonstrates Exceptional Performance With Cardiovascular Systems’ Diamondback 360® Coronary Orbital Atherectomy System (OAS) Dual Mode of Action
ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, announced today that results from a real-world optical coherence tomography (OCT) imaging study of coronary OAS patients were released at EuroPCR 2021. […]
TricValve® Transcatheter Bicaval Valves System Receives CE Mark
HONG KONG, May 20, 2021 /PRNewswire/ — OrbusNeich® Medical Company Ltd. and P&F Products & Features®, under the joint partnership OrbusNeich P&F, today announced that the TricValve® Transcatheter Bicaval Valves System has received CE mark approval. The TricValve® Transcatheter Bicaval Valve (bioprosthesis) is developed for the treatment of caval reflux present in cases of […]
Hydromer Selected as Key Coatings Provider for Avinger’s Image-Guided PAD Therapeutic Devices
Concord, NC, May 20, 2021 (GLOBE NEWSWIRE) — via NewMediaWire — Hydromer, Inc. (the “Company”) (HYDI:OTC) is proud to announce it has been selected as the key coatings and services partner for Avinger, Inc.’s intravascular image-guided, catheter-based systems for diagnosis and treatment of patients with Peripheral Artery Disease (PAD). Avinger’s best-in class product […]
Endologix LLC Announces Launch of ALTO® Abdominal Stent Graft System in Canada and Argentina
Company Expands Global Reach with Latest Endovascular Aneurysm Repair (EVAR) Technology IRVINE, Calif.–(BUSINESS WIRE)–Endologix LLC, a leader in the treatment of vascular disease, today announced the first implant of its ALTO® Abdominal Stent Graft in Canada following recent approval from Health Canada. ALTO was also recently approved for commercial sale […]
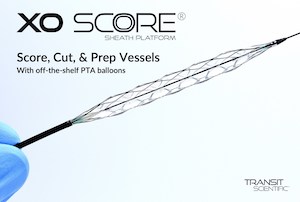
Cutting-Edge Scoring Sheath Platform Earns CE Mark Approval
New metal-alloy scoring sheath CE Mark cleared for treatment of peripheral artery disease and end stage renal disease. PARK CITY, Utah, May 10, 2021 /PRNewswire/ — Transit Scientific announced it received CE Mark clearance in the European Union for the XO Score® Scoring Sheath Platform to facilitate dilation of stenotic material in the peripheral vasculature […]