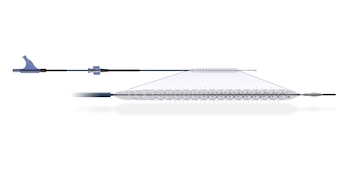
New Larger Implant Enables Focal Dissection Repair Across a Wider Range of Vessel Sizes WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced Food and Drug Administration PMA approval for the expansion of its Tack Endovascular System® (6F) portfolio. The new approved device […]
Peripheral/Endo
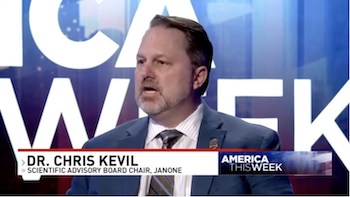
JanOne Scientific Advisory Board Chair and Leading Pain Expert Featured on ABC-7 WJLA America This Week
Dr. Christopher Kevil, pioneer in nitrite based PAD treatment and Dr. Amol Soin, foremost expert is neuropathic pain, provide scientific insight on the pain relieving qualities of JanOne’s lead drug candidate Interview to be distributed nationally on all 193 Sinclair broadcasting network affiliate stations in 89 markets across the nation […]
Ra Medical Systems Receives FDA IDE Approval to Begin Pivotal Atherectomy Clinical Study
CARLSBAD, Calif.–(BUSINESS WIRE)–Ra Medical Systems, Inc. (NYSE: RMED), a medical device company focused on commercializing excimer laser systems to treat vascular and dermatological diseases, announces approval from the U.S. Food and Drug Administration (FDA) that the Company has provided sufficient data to support initiating an investigational device exemption (IDE) to […]
Leading Cardiovascular Researcher And PAD Treatment Pioneer, Dr. Christopher Kevil, To Chair JanOne Scientific Advisory Board
Kevil brings global scientific expertise to advance JanOne’s drug development portfolio and advise on upcoming Phase 2b clinical trials for PAD LAS VEGAS, Jan. 16, 2020 /PRNewswire/ — JanOne Inc. (NASDAQ:JAN) appoints Christopher Kevil, Ph.D., a pioneer in nitrite based PAD treatment, to lead its scientific advisory board. In this role, Dr. Kevil will […]
Emboline Completes Enrollment in SafePass 2 Clinical Study of Emboliner Embolic Protection Catheter
Early Results from First 24 Patients Show Excellent Safety Profile and Technical Performance, with Significant Debris Capture and Removal in All Patients SANTA CRUZ, Calif.–(BUSINESS WIRE)–Emboline™, Inc., developer of total embolic protection technology for transcatheter aortic valve replacement (TAVR), today announced completion of enrollment in its SafePass™ 2 clinical study […]
San Angelo’s First Outpatient Cath Lab to Open
Dr. Milton Leon is opening the San Angelo Cardiovascular Center of Excellence to bring quality care, cost savings, and convenience to the area SAN ANGELO, Texas, Jan. 13, 2020 /PRNewswire/ — San Angelo is getting its first outpatient catheterization lab, bringing a new level of convenience, cost savings, and state-of-the art care to […]
PQ Bypass Announces 100th Patient in Clinical Study to Evaluate Percutaneous Fem-Pop Bypass for Extremely Long Blockages in Leg Arteries
DETOUR Procedure Designed to Move Treatment from Inpatient to Outpatient Setting MILPITAS, Calif.–(BUSINESS WIRE)–Silicon Valley-based medical device company PQ Bypass announced today the 100th patient in the DETOUR2 Clinical Trial, which evaluates the safety and effectiveness of the minimally invasive DETOUR procedure for percutaneous femoropopliteal bypass. The case was performed […]
FDA Grants Breakthrough Device Designation to Reflow Medical’s Temporary Spur Stent System
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical announces that the Temporary Spur Stent System, a novel retrievable stent technology intended for the treatment of below-the-knee (BTK) peripheral artery disease, has been designated for the Breakthrough Devices Program by the U.S. Food and Drug Administration (FDA). The Breakthrough Devices Program is designed to […]
Alucent Biomedical Announces FDA Approval to Proceed with Natural Vascular Scaffolding Clinical Trial
SALT LAKE CITY–(BUSINESS WIRE)–Alucent Biomedical Inc. has received U.S. Food and Drug Administration approval to proceed with a Phase 1 clinical trial to evaluate the safety and efficacy of its revolutionary Natural Vascular Scaffolding (NVS) technology. The therapy is designed to treat peripheral artery disease (PAD) of the lower extremities, a […]
Penumbra Announces FDA Clearance of Indigo® Aspiration System for Treatment of Pulmonary Embolism
ALAMEDA, Calif.–(BUSINESS WIRE)–Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced U.S. Food and Drug Administration 510(k) clearance for expanded indication of the Indigo® Aspiration System. As part of the Indigo Aspiration System, the Indigo Aspiration Catheters and Separators are indicated for the removal of fresh, […]