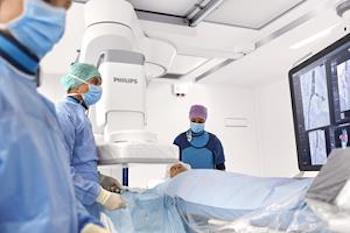
Industry-leading platform combines clinical excellence and workflow innovation, helping clinicians and hospitals to deliver outstanding patient care Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the one millionth procedure on its flagship Azurion image-guided therapy platform. Since its introduction in February 2017, Azurion […]
Peripheral/Endo
CryoLife Receives CE Mark for E-nside Thoraco-abdominal Stent Graft
First Off-the-Shelf Thoraco-abdominal Stent Graft with Pre-Cannulated Inner Branch Technology ATLANTA, Dec. 2, 2019 /PRNewswire/ — CryoLife, Inc. (NYSE: CRY), a leading cardiac and vascular surgery company focused on aortic disease, announced today that it has received CE Mark for the E-nside TAAA multibranch stent graft system for the endovascular treatment of thoraco-abdominal aneurysms. Approximately […]
CryoLife Receives CE Mark for E-nya Thoracic Stent Graft
Product Launch Anticipated in the First Quarter 2020 ATLANTA, Dec. 2, 2019 /PRNewswire/ — CryoLife, Inc. (NYSE: CRY), a leading cardiac and vascular surgery company focused on aortic disease, announced today it has received CE Mark for the E-nya thoracic stent graft system for the minimally invasive repair of lesions of the descending thoracic aorta, […]
One millionth procedure carried out on Philips Azurion advanced image-guided therapy platform
November 27, 2019 Industry-leading platform combines clinical excellence and workflow innovation, helping clinicians and hospitals to deliver outstanding patient care Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the one millionth procedure on its flagship Azurion image-guided therapy platform. Since its introduction […]
InspireMD Reports Updated Positive CGuard™ EPS Data Presented at VEITH 2019
CGuard™ EPS clinical data featured as a prominent discussion point in multiple key presentations Data from investigator-initiated multicenter, 729-patient IRONGUARD 2 study suggests that the use of CGuard™ EPS in routine clinical practice is associated with no major periprocedural, 30-day or one-year neurological complications TEL AVIV, Israel, Nov. 26, 2019 […]
JanOne Acquires Worldwide, Exclusive License for Promising Treatment of Peripheral Arterial Disease (PAD)
Phase 2b testing planned for PAD treatment and PAD-associated pain to address 8.5 million patient US market LAS VEGAS, Nov. 25, 2019 /PRNewswire/ — JanOne Inc. (NASDAQ: JAN), a company focused on reducing opioid addiction by finding treatments for conditions accompanied by pain and bringing to market drugs and therapies with nonaddictive pain-relieving properties, […]
Favorable Outcomes with TCAR vs. Carotid Endarterectomy in Patients with Carotid Artery Disease
Updated Results of TCAR Surveillance Project Presented at 2019 VEITHsymposium NEW YORK, New York – November 22, 2019 – Silk Road Medical, Inc. (Nasdaq: SILK), a company focused on reducing the risk of stroke and its devastating impact, today announced the presentation of real-world data for the treatment of […]
Data Show Zilver® PTX® Leads to Fewer Complications and Shorter Hospital Stays Than Traditional Bypass Surgery
BLOOMINGTON, Ind.–(BUSINESS WIRE)–At this year’s VEITHsymposium®, Dr. Marc Bosiers presented data that show that patient treatment with the Zilver® PTX® stent has several benefits when compared to traditional bypass surgery. The data, which were gathered from a randomized controlled trial, show that treatment with Zilver PTX results in fewer complications and shorter […]
Medtronic Drug-Coated Balloon Receives U.S. FDA Approval to Treat Arteriovenous Fistula Lesions
Clinical Data Demonstrates IN.PACT™ AV DCB Is Safe, Reduces Reinterventions, and Maintains Access for End-Stage Renal Disease Patients Undergoing Dialysis DUBLIN, Nov. 21, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) approval of the IN.PACT™ AV drug-coated balloon (DCB), a paclitaxel-coated balloon indicated for the […]
Merit Medical Receives FDA Breakthrough Device Designation for WRAPSODY™ Endovascular Stent Graft System
SOUTH JORDAN, Utah, Nov. 21, 2019 (GLOBE NEWSWIRE) — Merit Medical Systems, Inc. (NASDAQ: MMSI), a leading manufacturer and marketer of proprietary disposable devices used in interventional, diagnostic and therapeutic procedures, particularly in cardiology, radiology, oncology, critical care and endoscopy, announced today that it has been granted Breakthrough Device Designation […]