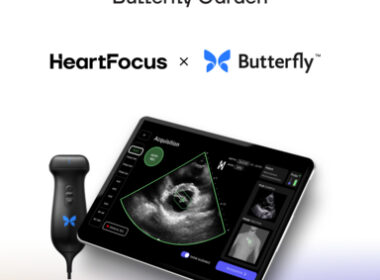
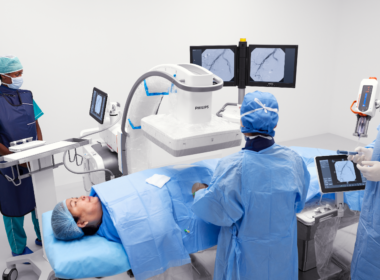
Leveraging Butterfly Network’s platform, UltraSight seeks to enable patients to access cardiac imaging with real-time AI guidance
TEL AVIV, Israel, June 27, 2024 /PRNewswire/ — Today, UltraSight, a digital health pioneer transforming cardiac imaging through the power of artificial intelligence, announced it has joined Butterfly Garden, an artificial intelligence (AI) Marketplace launched by Butterfly Network, Inc. (“Butterfly”) (NYSE: BFLY), a digital health company transforming care through the power of portable, semiconductor-based ultrasound technology and intuitive software.
UltraSight and Butterfly have partnered to increase patient access to cardiac care by enabling more healthcare professionals to perform cardiac ultrasound. Subject to regulatory approvals and authorizations, UltraSight aims to integrate and deploy its real-time AI guidance software on Butterfly’s imaging platform and build the software for use with Butterfly’s single-probe, whole-body handheld ultrasound system.
Cardiovascular disease (CVD) is the leading global cause of death, accounting for an estimated 18 million deaths yearly. Today patients face significant delays in receiving crucial cardiac testing due to a fragmented market full of system bottlenecks, in addition to a national shortage of expert sonographers.
UltraSight’s mission is to empower any medical professional, including novice users with no prior sonography experience, to confidently conduct echocardiographic examinations and capture diagnostic quality cardiac ultrasound images at the point of care. Achieving compatibility with the most prominent handheld ultrasound device companies in the market, such as Butterfly, facilitates the company’s goal of increasing access to cardiac care and reducing system bottlenecks for patients.
“Joining the Butterfly Garden marks an important moment in our mission to revolutionize cardiac care,” said Davidi Vortman, CEO of UltraSight. “By integrating our real-time AI guidance software with Butterfly’s cutting-edge ultrasound technology, we are poised to transform the landscape of cardiac imaging. This collaboration will empower healthcare professionals, regardless of their experience level, to perform accurate and timely cardiac ultrasound exams at the point of care. Together, we will break down existing barriers in cardiac care, ensuring that more patients receive the critical diagnostics they need, when and where they need it most.”
“We are thrilled to partner with UltraSight to bring their AI guidance software to Butterfly devices in an effort to mitigate the pressing issue of sonographer shortages, among other workforce challenges, impacting medical communities around the world,” said Darius Shahida, chief strategy officer of Butterfly Network. “UltraSight and Butterfly are jointly dedicated to making healthcare more efficient, effective and accessible through high-quality ultrasound that’s easy-to-use and globally available.”
In August 2023, Butterfly Network launched Butterfly Garden, allowing third-party developers access to its proprietary SDK and APIs to build new AI applications that work in conjunction with Butterfly’s imaging platform, bringing with it access to the largest point-of-care ultrasound customer base. UltraSight, with capabilities to provide more medical professionals with the ability to take high-quality diagnostic images of the heart, has the potential to close the gap between novice and skilled Butterfly ultrasound users, and is an ideal match for the program.
When paired with ultrasound devices, and following the appropriate regulatory clearance for each device, UltraSight’s underlying AI neural network can predict the position of the ultrasound probe relative to the heart based on the ultrasound video stream and guide the user on maneuvering the probe to capture diagnostic quality cardiac images.
UltraSight’s partnership with Butterfly follows a series of recent collaborations with other industry leaders such as Mayo Clinic and EchoNous. For more information about UltraSight, visit www.ultrasight.com. For more information about Butterfly, visit www.butterflynetwork.com.
About UltraSight
UltraSight’s mission is to make diagnostic imaging more accessible by empowering medical professionals to successfully acquire timely and accurate cardiac ultrasound images anywhere. UltraSight’s AI-driven software offers real-time guidance, making cardiac ultrasound accessible and efficient, which may lead to quicker diagnoses and improved patient care. In 2022, UltraSight won the Bristol Myers Squibb Improving Cardiovascular Disease Outcomes Challenge as the most “innovative cardiac technology.” Additionally, the company was awarded a patent for its real-time guidance solution for ultrasound devices. UltraSight’s software has FDA 510(k) Clearance, is UKCA and CE Marked, and has Israeli AMAR Clearance to assist medical professionals in performing cardiac ultrasound scans. For more news and information, visit our website or follow UltraSight on LinkedIn and Twitter
About Butterfly Network
Founded by Dr. Jonathan Rothberg in 2011, Butterfly Network is a digital health company with a mission to democratize medical imaging by making high-quality ultrasound affordable, easy-to-use, globally accessible, and intelligently connected, including for the 4.7 billion people around the world lacking access to ultrasound. Butterfly created the world’s first handheld single-probe, whole-body ultrasound system using semiconductor technology, Butterfly iQ. The company has continued to innovate, leveraging the benefits of Moore’s Law, to launch its second-generation Butterfly iQ+ in 2020, and third-generation iQ3 in 2024 – each with increased processing power and performance enhancements. The disruptive technology has been recognized by TIME’s Best Inventions, Fast Company’s World Changing Ideas, CNBC Disruptor 50, and MedTech Breakthrough Awards, among other accolades. With its proprietary Ultrasound-on-Chip™ technology, intelligent software, and educational offerings, Butterfly is paving the way to mass adoption of ultrasound for earlier detection and remote management of health conditions around the world. Butterfly devices are commercially available to trained healthcare practitioners in areas including, but not limited to, parts of Africa, Asia, Australia, Europe, the Middle East, North America and South America; to learn more about available countries, visit: www.butterflynetwork.com/choose-your-country.
SOURCE UltraSight
Radiology
Data Supporting Nanox.AI Cardiac Solution to Be Presented at 19th Annual Meeting of the Society of Cardiovascular Computed Tomography
PETACH TIKVA, Israel, June 25, 2024 (GLOBE NEWSWIRE) — NANO-X IMAGING LTD (“Nanox” or the “Company,” Nasdaq: NNOX), an innovative medical imaging technology company, today announced that data from five studies supporting the AI Cardiac Solution (HealthCCSng) of its subsidiary, Nanox AI, will be presented at the 19th Annual Meeting of the Society of Cardiovascular Computed Tomography (SCCT), being held July 18-21 in Washington, D.C. The studies are being presented by esteemed institutions Brigham & Women’s Hospital, Einstein Jefferson Medical Center, Corewell Health, Massachusetts General Hospital and Beilinson Medical Center. Nanox AI’s Cardiac Solution (HealthCCSng) utilizes medical imaging data from routine chest CT scans to automatically quantify coronary artery calcium (CAC) levels. CAC is known as a strong predictor of future cardiovascular events; patients in the highest CAC category are up to 6x more likely to suffer cardiac events. The abstract details are as follows: Title: The Frequency, Prevalence, And Outcomes of Incidentally Detected Coronary Artery Calcium Using Artificial Intelligence Analysis Among Patients with Immune Mediated Inflammatory Diseases (Best Abstract Award Finalist)Lead Author: Brittany Weber, Brigham & Women’s HospitalAbstract number: 2024-A-817-SCCTPresentation date and time: Saturday, July 20, 10:30 AM, Liberty I-M Title: Optimizing Preventive Cardiology: Harnessing AI for Early Detection of Coronary Artery DiseaseLead author: Dr. Chiduzie Madubata, Einstein Jefferson Medical CenterAbstract number: 2024-A-573-SCCTPresentation date and time: Friday, July 19, 9:30AM – 10:15AM, Exhibit Hall Title: AI Empowering Early Detection of CAD Patients for Improved Cardiac CareLead author: Dr. David Langholz, Corewell HealthAbstract number: 2024-A-641-SCCTPresentation date and time: Friday, July 19, 9:30AM – 10:15AM, Exhibit Hall Title: Artificial-intelligence Based Detection of Coronary Artery Calcium on Chest CT to Enhance Cardiovascular Risk Assessment of Individuals with Elevated Lipoprotein (a)Lead author: Milena Petranovic, Massachusetts General HospitalAbstract number: 2024-A-820-SCCTPresentation date and time: Friday, July 19, 9:30AM – 10:15AM, Exhibit Hall Title: Opportunistic Screening of Coronary Artery Calcification On Non-gated Conventional CT Scans Using Artificial IntelligenceLead author: Dr. Ashraf Hamdan, Beilinson HospitalAbstract number: 2024-A-532-SCCTPresentation date and time: Saturday, July 20, 9:30AM – 10:15AM, Exhibit Hall Throughout SCCT, Nanox AI’s representative will also be available for meetings in the Honeysuckle Meeting Room. You can schedule a meeting here. About Nanox AINanox AI is the deep-learning medical imaging analytics subsidiary of Nanox. Nanox AI’s solutions are developed to target highly prevalent chronic and acute diseases affecting large populations around the world. Leveraging AI technology, Nanox AI helps clinicians extract valuable and actionable clinical insights from routine medical imaging that otherwise may go unnoticed, potentially initiating further medical assessment to establish individual preventative care pathways for patients. For more information, please visit https://www.nanox.vision/ai. About NanoxNanox (NASDAQ: NNOX) is focused on applying its proprietary medical imaging technology and solutions to make diagnostic medicine more accessible and affordable across the globe. Nanox’s vision is to increase access, reduce costs and enhance the efficiency of routine medical imaging technology and processes, in order to improve early detection and treatment, which Nanox believes is key to helping people achieve better health outcomes, and, ultimately, to save lives. The Nanox ecosystem includes Nanox.ARC— a multi-source Digital Tomosynthesis system that is cost-effective and user-friendly; an AI-based suite of algorithms that augment the readings of routine CT imaging to highlight early signs often related to chronic disease (Nanox.AI); a cloud-based infrastructure (Nanox.CLOUD); and a proprietary decentralized marketplace, through Nanox’s subsidiary, USARAD Holdings Inc., that provides remote access to radiology and cardiology experts; and a comprehensive teleradiology services platform (Nanox.MARKETPLACE). Together, Nanox’s products and services create a worldwide, innovative, and comprehensive solution that connects medical imaging solutions, from scan to diagnosis. For more information, please visit www.nanox.vision. Contacts Media Contact:Ben ShannonICR WestwickeNanoxPR@icrinc.com Investor Contact:Mike CavanaughICR Westwickemike.cavanaugh@westwicke.com
HeartFocus Joins Butterfly Garden to Develop Software Enabling Any Healthcare Professional to Conduct Lifesaving Echos on Butterfly’s Imaging Platform
June 24, 2024 07:00 AM Eastern Daylight Time BORDEAUX, France–(BUSINESS WIRE)–HeartFocus, the revolutionary, AI-driven heart exam software by DESKi, a provider of automated, accurate, and data-driven image analysis tools today announced it has joined Butterfly Garden, an artificial intelligence (AI) Marketplace launched by Butterfly Network, Inc., (“Butterfly”) (NYSE: BFLY) a digital health […]
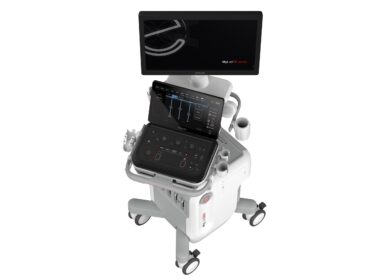
At the SIRM Congress 2024 in Milan, ESAOTE presents the brand-new MyLab™E80, an E-series ultrasound device designed for professionals dealing with more complex clinical cases
The unveiling took place at the Esaote stand (I-03), where industry professionals could find out more about the features and performance of the new system, throughout the Congress. MILAN, June 21, 2024 /PRNewswire/ — Esaote – a leading Italian company in the field of medical imaging – has unveiled its new MyLab™E80 ultrasound device to the world of radiology. The unveiling took place at the 51st SIRM Congress 2024 (Allianz MiCo Convention Center, Milan – Stand I-03), the first time the three scientific societies in the field of radiology (SIRM, AIMN, AIRO) have come together for their first joint Congress, entitled “The Next Generation”.
Continue Reading
The new Esaote MyLab™E80 ultrasound device
Another step forward in terms of innovation, MyLab™E80 is the first ultrasound system in Esaote’s brand-new E series. The ‘E’ stands for Expertise, referring to a series of devices designed for professionals who deal with more complex clinical cases, where diagnostic certainty and clinical efficiency play a decisive role.
This ultrasound scanner guarantees intuitive workflow management, with advanced A.I.-powered automation features to speed up repetitive operations and simplify complex functions. Doctors can therefore conduct more thorough examinations based on images with a very high level of detail. The system also comes with fusion imaging technology, for the assessment of liver disease and breast lesions.
MyLab™E80 integrates the Augmented Insight™ functions of the MyLab™X90 and provides users with options of a conventional or touchscreen control panel, in line with the innovative selection launched in the A Series. “The agile and fast MyLab™E80 and its versatile and mobile advanced features embodies our new vision as part of our ongoing commitment to providing high-quality healthcare. We have therefore improved the reproducibility, efficiency and diagnostic accuracy of our ultrasound scanners,” stated Mariagrazia Bella, Marketing Director for Italy. “MyLab™E80 is designed to adapt to any healthcare environment. With five probe connectors and custom configurations, it’s ideal for both routine and advanced examinations. The battery offers autonomous scanning at full power, while the modern touch panel simplifies cleaning procedures, ensuring adaptability even in the most demanding environments.”MyLab™E80 completes the renewal of the range of ultrasound scanners developed by Esaote, alongside the MyLab™X90, MyLab™A70 and MyLab™A50. In line with its new brand identity and slogan of “Health with Care”, Esaote confirms its mission and the sense of care and empathy with which it approaches its everyday work, to improve people’s well-being. www.esaote.com Esaote Group Leader in medical imaging (ultrasound, MRI, software to manage the diagnostic process). At the end of 2023, the Group had 1,250 employees, half of whom in Italy. With facilities in Genoa and Florence, and its own production and research units in Italy and the Netherlands, Esaote maintains a presence in over 100 countries. www.esaote.com© Copyright Esaote 2024Photo – https://mma.prnewswire.com/media/2442819/Esaote_SpA.jpgLogo – https://mma.prnewswire.com/media/2323931/4771337/ESAOTE_Logo.jpg
RADPAIR and NewVue Announce Groundbreaking Partnership to Enhance Radiologist Wellbeing and Workflow Efficiency
TAMPA, Fla. and KNOXVILLE, Tenn., June 18, 2024 /PRNewswire/ — RADPAIR and NewVue are excited to announce a strategic partnership that will be the first of its kind in the radiology industry. This collaboration integrates RADPAIR’s advanced AI diagnostic reporting capabilities into NewVue’s workflow orchestrator, creating the industry’s first cloud-native solution to enhance radiologists’ well-being and job satisfaction while improving workflow and report quality.
The combined solutions will transform how radiologists and practices interact with their worklists, manage clinical information, dictate reports, follow up on cases, and track productivity. Leveraging modern capabilities like AI and large language models (LLMs), this next-generation cloud product delivers an innovative, efficient, and enjoyable experience.
EmpowerSuite automatically generates a curated worklist based on the radiologist’s profile, considering their preferred specialties, credentialing, insurance coverage, scheduled shifts, and physical location. This next-generation approach to workload distribution and management departs from traditional first-generation worklists that require extensive maintenance of rules and lists. Radiologists can also adjust the reading pace and cadence based on their mood, improving job satisfaction.
Studies opened from the worklist launch PACS and the EmpowerSuite Clinical information screen. This “radiologist cockpit” uses AI to summarize all available prior reports and clinical information, saving radiologists time by eliminating the need to search multiple systems. The cockpit also hosts the RADPAIR AI-powered reporting system, which generates reports from conversational inputs. Automatic reporting and dynamic editing features allow for near real-time report generation and simple click-and-drag editing for minor revisions. The cloud-native platform ensures easy sign-in with zero context switching, allowing radiologists to focus entirely on diagnostics. This innovative workflow and reporting system integrates with existing PACS and replaces legacy VR and dictation systems.
“We’ve been really thinking about how to make the lives of radiologists fun again while increasing quality. Our new cutting-edge classification system PAIR Insights allows radiologists to seamlessly insert guidelines into reports, enhancing understanding for both clinicians and patients. This partnership with NewVue underscores our commitment to improving the radiology experience,” said Dr. Avez Rizvi, Founder & CEO of RADPAIR.
“We are excited to join forces with RADPAIR to create a solution that enhances the efficiency of radiology reporting and significantly improves the well-being of radiologists. By integrating RADPAIR’s cutting-edge technology into our workflow orchestrator, we are setting a new standard for radiology practices,” said Kyle Lawton, CEO of NewVue.
###
About RADPAIR:RADPAIR is a leader in innovative radiology solutions, dedicated to improving the radiologist’s experience and the quality of patient care. With a focus on smart systems and trusted guidelines, RADPAIR sets the standard in radiology reporting.
About NewVue:NewVue specializes in workflow orchestration for healthcare providers, delivering solutions that enhance operational efficiency and care quality. NewVue’s platform integrates seamlessly with existing systems to optimize workflows and improve patient outcomes.
Media ContactFatima BaigRADPAIR310-766-8911[email protected]
SOURCE RADPAIR
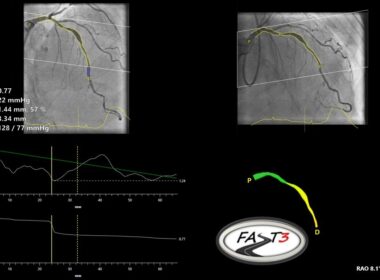
Pie Medical Imaging announces enrollment completion of the FASTIII clinical trial
MAASTRICHT, Netherlands, June 18, 2024 /PRNewswire/ — Pie Medical Imaging, a global leader in cardiac imaging, announced the completion of enrollment in FASTIII, a multi-center randomized clinical trial, which investigates the use of angiography-based vessel fractional flow reserve (CAAS vFFR) in patients undergoing coronary revascularization procedures. vFFR can assess whether a coronary artery narrowing is functionally significant and may require revascularization.
Continue Reading
CAAS vFFR Software
FASTIII is the largest non-inferiority trial running (having enrolled 2228 patients), in which an angiographically derived vFFR guided strategy is compared to a FFR guided strategy to guide coronary revascularization. The primary endpoint is a composite of all-cause death, any myocardial infarction, or any revascularization at 1-year post randomization.
Following the tremendous efforts of the principal investigator, Dr Joost Daemen (cardiologist at Thoraxcenter at the Erasmus University Medical Center, Rotterdam, The Netherlands), over 35 participating centers and ECRI (sponsor of the trial), the patient enrollment has concluded.
“The FASTIII trial is aimed to establish the role of vFFR to coronary revascularization in patients with intermediate coronary artery lesions. An important milestone was reached Friday, May 31st, 2024, when the last patient was enrolled”, said Joost Daemen Principal Investigator of the trial. “The next phase will consist of close follow-up of all patients who generously agreed to participate in the important trial. We hope to present our findings by the end of 2025″. Additionally,”PMI is committed to providing clinicians and patients with long-term coronary data to inform their treatment decisions, ” said René Guillaume, Managing Director at Pie Medical Imaging. “Our prior studies have shown diagnostic accuracy and reproducibility of vFFR calculation”. FASTIII will establish its role in routine clinical practice. The trial is funded by research grants from Pie Medical Imaging (Maastricht, The Netherlands) and Siemens Healthineers (Erlangen, Germany). The study is sponsored by ECRI (European Cardiovascular Research Institute, Rotterdam-the Netherlands). Cardialysis (Rotterdam, The Netherlands) is responsible for trial services including trial management and Core Laboratory activities.About Pie Medical ImagingPie Medical Imaging BV is a world leader in analysis and visualization of cardiovascular images. In Maastricht (The Netherlands), it hosts the global sales for the CAAS and 3mensio product lines.PMI and 3mensio Medical Imaging part of the Esaote Group, leader in the biomedical equipment sector. More information about PMI is available at www.piemedicalimaging.com Photo – https://mma.prnewswire.com/media/2438118/CAAS_vFFR.jpgLogo – https://mma.prnewswire.com/media/2438117/PMI_logo.jpg
EchoNous Announces Sale of 157 Units of Kosmos Ultrasound Technology to Madrid’s Primary Care System
MADRID, Spain, June 17, 2024 (GLOBE NEWSWIRE) — EchoNous, a leader in AI-enabled point-of-care ultrasound systems (POCUS), is excited to announce the installation of 157 Kosmos units in more than 150 primary care centers across Madrid, Spain. Known for its user-friendly design, the handheld Kosmos system simplifies access to diagnostic-grade imaging, enabling users of various experience levels to perform accurate ultrasound assessments. The installation aligns with Spain’s initiative to expand the capabilities of its primary care centers and reduce hospital congestion. Traditionally, hospitals have been the sole source of ultrasound examinations for Spanish citizens. As a result, many hospitals have become overcrowded, often forcing new patients to wait several months to receive diagnoses for myriad conditions. By empowering primary care physicians with Kosmos, Madrid aims to make primary care centers the first stop for patients requiring ultrasound examinations, removing pressure from hospitals. Kosmos offers numerous advanced features such as AI-driven anatomical labeling and precise view identification, allowing the user to examine the patient and determine whether a hospital visit is necessary in minutes. Administering ultrasound examinations as early as possible also increases the efficiency of Madrid’s healthcare system. Patients who are confirmed healthy will be diverted from hospitals while patients who require further investigation will face fewer delays between vital examinations. “Kosmos helps family physicians perform ultrasounds with remarkable simplicity,” said a Madrid physician involved in the Kosmos rollout. “The equipment brilliantly supports the core objectives of introducing ultrasounds to Madrid’s primary care space: reducing unnecessary patient referrals, and optimizing care for people who actually need hospital service.” The implementation of Kosmos positions Spain as a leader in the global adoption of portable ultrasound imaging. In addition to expanding access to ultrasound services, the technology provides AI-driven guidance to assist each user’s formal ultrasound training. “At EchoNous, our vision is to put Kosmos in the hands of more healthcare professionals while shortening the path of becoming proficient with ultrasound technology,” said Graham Cox, CEO of EchoNous. “This partnership is going to bring advanced diagnostic capabilities to millions of people, redefining the concept of primary care as we know it.” About EchoNous: Based in Redmond, Washington, EchoNous is reshaping the landscape of point-of-care ultrasound. By fusing unparalleled ultrasound performance with industry-leading AI, EchoNous provides clinicians with rapid, accurate insights, improving patient outcomes across healthcare environments. For more information, visit www.echonous.com. Luke Baldwin Vice President Global Marketing luke.baldwin@echonous.com
Philips Zenition 90 Motorized receives FDA 510(k) clearance, helping clinicians deliver high quality care with a high-powered and fast motorized mobile C-arm
June 17, 2024 Intuitive motorization for greater control and high-power (25 kW [1]) for state-of-the-art image quality supports complex vascular needs and a full range of clinical proceduresAutomated workflows contribute to greater flexibility and independence for clinicians, providing more time to focus on achieving the best possible outcomes for patients Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Philips Image Guided Therapy Mobile C-arm System 9000 – Zenition 90 Motorized, designed to help clinicians deliver high-quality care to more patients. Philips is partnering with its customers to improve productivity. The new mobile C-arm with expanded capabilities is designed to meet complex vascular needs, but also a range of clinical procedures such as cardiac interventions, pain management and urology. Philips will be showcasing its newly introduced mobile C-arm at the 2024 Society for Vascular Surgery Annual Meeting, June 19-22, in Chicago. Increased control and efficiency with automated workflowsThe Philips Zenition Image-Guided Therapy Mobile C-arm Systems bring together innovations in image capture and processing, ease-of-use, and versatility, many of which were pioneered on Philips’ highly successful image guided therapy platform Azurion. Motorized and impressively fast, the Zenition 90 Motorized is an intuitive C-arm that allowsclinicians to control it from the table-side with user-friendly controls and time-saving features – empowering the clinician with greater flexibility and independence. It delivers state-of-the-art image quality for the most challenging procedures and is designed to meet complex procedural needs. The system allows greater clinical efficiency thanks to its automated workflows, the image controls via the Touch Screen Module and the advanced software solutions. “During complex procedures, it’s vital to be able to rely on surgical imaging systems. As clinicians navigate their way through challenging anatomy, the priority is to quickly visualize small anatomical details while limiting X-ray dose,” said Mark Stoffels, Business Leader Philips Image Guided Therapy Systems. “The new Zenition 90 Motorized empowers medical teams to confidently perform a wide range of interventions while achieving the best possible outcome for their patients.” In independent hands-on usability studies of clinicians from the US and EU with the Zenition 90 Motorized in simulated environments; 100% of users said that with the Table Side Operator, they have complete control over C-arm movements [2] and 97% report that workflow features such as Automatic Vascular Outlining will help save time during procedures [3]. As part of its commitment to sustainability and providing customers with responsible choices, Philips leveraged its EcoDesign process for the Zenition 90 Motorized to improve product life by 25% and power efficiency by 13% [4]. Philips latest image guided therapy mobile C-arm system is also available in a non-motorized configuration.[1]Also available in 15 kW[2] Results obtained during claims substantiation study performed in June 2022 and May 2023 by Use-Lab GmbH, an independent company. Response is based on 25 physicians from the EU and the US, who answered a questionnaire after a usability study with additional hands-on time with the system.[3] Results obtained during claims substantiation study performed in June 2022 and May 2023 by Use-Lab GmbH, an independent company. Response is based on 49 clinicians from the EU and the US, who answered a questionnaire after a usability study with additional hands-on time with the system.[4] Compared to its predecessor, Zenition 70- Zenition 90 Motorized and Zenition 90 are available for sales in a limited number of countries.- Some features are optional for Zenition 90 Motorized and Zenition 90.- Actual product representation may vary. For further information, please contact:Joost MalthaPhilips External RelationsTel. : +31 6 10558116E-mail: joost.maltha@philips.com About Royal PhilipsRoyal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2023 sales of EUR 18.2 billion and employs approximately 69,100 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachments
Positron Corporation Joins Cardiac PET Industry Coalition (CPIC)
Niagara Falls, NY, June 14, 2024 (GLOBE NEWSWIRE) — Positron Corporation (“Positron” or the “Company”) (OTC: POSC), a leading molecular imaging medical device company offering PET and PET-CT (Positron Emission Tomography/Computed Tomography) imaging systems and clinical services, is pleased to announce its membership in the national alliance “Cardiac PET Industry Coalition” (CPIC), an organization at the forefront of quality of patient care, advocacy and innovation to advance the field of cardiovascular PET imaging. Positron joins forces with the coalitions founding members, Bracco Diagnostics, CDL Nuclear Technologies and Siemens Healthcare who have come together to promote federal policies that advance health outcomes for patients with cardiovascular disease and improve the availability of cardiac PET diagnostics throughout the United States. The CPIC will be a leading advocate for fair and transparent coverage and reimbursement policies that facilitate access to cardiac PET, align practitioners with regard to research opportunities, highlight the clinical and economic benefit of the modality, and provide expanded opportunities to educate healthcare providers and policymakers on the overall value of cardiac PET. CPIC has begun its work by weighing in on 2024 final payment rules, engaging with stakeholders in the cardiac community, and developing a 2024 policy agenda. Adel Abdullah, President of Positron stated, “Positron welcomed the opportunity to join the coalition, and personally, and I am honored to be part of current and future CPIC committees focused on the education, availability and advancement of the PET modality for cardiac studies. We believe Positron’s PET/PET-CT technology and ability to deliver the best value in the industry will play a key role in the adoption and growth of cardiac PET. Positron and our team will support and serve the coalition as best as possible for the future of the industry, concluded Mr. Abdullah.” Cardiac PET Industry Coalition CPIC passionately advocates for and protects the reimbursement of Cardiac PET, ensuring equitable access to innovative and life-saving technologies for healthcare providers and patients. For more information please visit www.cpicoalition.com About Positron Corporation Positron Corporation is a medical technology company that co-develops, manufactures, and sells state-of-the-art PET and PET-CT imaging systems and clinical services to nuclear medicine healthcare providers throughout North America. Positron specializes in the field of cardiac Positron Emission Tomography (PET) imaging, the gold standard in cardiac diagnostics. Positron’s innovative PET/PET-CT technologies, clinical services and practice solutions enables healthcare providers to accurately diagnose coronary artery disease and improve patient outcomes while practicing cost effective medicine. Positron’s Attrius® PET and NeuSight PET-CT imaging systems and distinct market position are substantial advantages unique to Positron that will facilitate the adoption of cardiac PET and the growth of the nuclear imaging market. Positron will soon offer a state-of-the-art PET-CT 4D molecular imaging device in the Affinity PET-CT 4D 64-Slice. Positron’s PET-CT(s) will enable nuclear cardiologists to utilize the full capabilities of molecular imaging and nuclear medicine. Positron’s PET-CT systems will also enable the Company to fully service and meet the demands of the vast oncology imaging segment of nuclear medicine. Positron is committed to expanding the cardiac and oncology PET modality by delivering the best technology and value to imaging specialists and will continue to advance its technology through its co-developer, supplier, and R&D venture with Shenyang Intelligent Neuclear Technology Co. a subsidiary of Neusoft Medical Systems. For more information please visit www.positron.com Forward-Looking Statements This press release contains statements which may constitute “forward-looking statements” within the meaning of the Securities Act of 1933 and the Securities Exchange Act of 1934, as amended by the Private Securities Litigation Reform Act of 1995. Those statements include statements regarding the intent, belief or current expectations of Positron Corporation, and members of its management as well as the assumptions on which such statements are based. Prospective investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and that actual results may differ materially from those contemplated by such forward-looking statements. The Company undertakes no obligation to update or revise forward-looking statements to reflect changed assumptions, the occurrence of unanticipated events or changes to future operating results even if new information becomes available in the future. FOR FURTHER INFORMATION, please visit the company’s website at www.positron.com, or contact: investor@positron.com
Nanox Launches Artificial Intelligence Functionality in Second Opinions Platform
Nanox integrates FDA-cleared population health AI solutions into its Second Opinions service New AI capabilities available to patients who submit chest and abdominal CT scans for second opinions Offering the power of Artificial Intelligence to their second opinion CT report to promote early detection of chronic conditions PETACH TIKVA, Israel, June 05, 2024 (GLOBE NEWSWIRE) — NANO-X IMAGING LTD (“Nanox” or the “Company,” Nasdaq: NNOX), an innovative medical imaging technology company, today announced that its deep-learning medical imaging analytics subsidiary, Nanox AI Ltd., has launched an artificial intelligence (AI) functionality in the Second Opinions online medical consultation service. Second Opinions is a platform provided by USARAD Holdings INC, a subsidiary of Nano-X Imaging Ltd., that provides teleradiology services. The platform connects patients with radiologists and other subspecialty physicians for additional consultation on their medical diagnoses. Second Opinions has integrated three of Nanox.AI’s FDA 510(k)-cleared AI solutions, enabling patients to conveniently get second opinions from experts in various medical and surgical subspecialties including radiology, neurology, oncology and orthopedic surgery. The integration of Nanox.AI’s tools is intended to promote the early detection of chronic conditions on chest and abdominal CT scans: AI Cardiac solution (HealthCCSng) detects coronary artery calcium, an early sign of coronary artery diseaseAI Bone solution (HealthOST) assesses bone mineral density, and highlights vertebral spine compression fractures which can indicate risk of osteoporosisAI Liver solution (HealthFLD) measures liver density, which can indicate fatty liver disease These AI-driven insights are reviewed and approved by Second Opinions physicians and incorporated into reports for patients who submit eligible chest and abdominal CT scans. “We are excited to bring AI-powered, early detection through the Second Opinions platform to patients seeking peace of mind concerning their health and diagnoses,” said Erez Meltzer, Nanox Chief Executive Officer. “The integration of Nanox.AI’s solutions into the Second Opinions service will help empower radiologists and other healthcare providers by providing them with advanced AI tools that aim to improve patient outcomes. We will continue exploring opportunities to leverage our AI technology to promote accessible early diagnosis and preventative management.” Learn more about Second Opinions and its new AI capabilities at Artificial Intelligence (AI) Service – Second Opinions. About USARADUSARAD is a U.S.-based teleradiology company with a network of radiologists, certified by the American Board of Radiology. USARAD provides imaging interpretation and database services to radiology practices, hospitals, medical clinics, diagnostic imaging centers, urgent care facilities and multi-specialty physician groups in the U.S and additional countries, improving service levels, streamlining practice economics and enhancing physician efficiency. About Nanox.AINanox AI is the deep-learning medical imaging analytics subsidiary of Nanox. Nanox.AI solutions are developed to target highly prevalent chronic and acute diseases affecting large populations around the world. Leveraging AI technology, Nanox AI helps clinicians extract valuable and actionable clinical insights from routine medical imaging that otherwise may go unnoticed, potentially initiating further medical assessment to establish individual preventative care pathways for patients. For more information, please visit https://www.nanox.vision/ai. About NanoxNanox (NASDAQ: NNOX) is focused on applying its proprietary medical imaging technology and solutions to make diagnostic medicine more accessible and affordable across the globe. Nanox’s vision is to increase access, reduce costs and enhance the efficiency of routine medical imaging technology and processes, in order to improve early detection and treatment, which Nanox believes is key to helping people achieve better health outcomes, and, ultimately, to save lives. The Nanox ecosystem includes Nanox.ARC— a multi-source Digital Tomosynthesis system that is cost-effective and user-friendly; an AI-based suite of algorithms that augment the readings of routine CT imaging to highlight early signs often related to chronic disease (Nanox.AI); a cloud-based infrastructure (Nanox.CLOUD); and a proprietary decentralized marketplace, through Nanox’s subsidiary, USARAD Holdings Inc., that provides remote access to radiology and cardiology experts; and a comprehensive teleradiology services platform (Nanox.MARKETPLACE). Together, Nanox’s products and services create a worldwide, innovative, and comprehensive solution that connects medical imaging solutions, from scan to diagnosis. For more information, please visit www.nanox.vision. Contacts Media Contact:Ben ShannonICR WestwickeNanoxPR@icrinc.com Investor Contact:Mike CavanaughICR Westwickemike.cavanaugh@westwicke.com