Revolutionizing radiology with cutting-edge imaging, smart automation, and effortless control — the Resona A20 is built to elevate diagnostic confidence like never before. MAHWAH, N.J., Dec. 1, 2025 /PRNewswire/ — Mindray, a global leader in healthcare technologies and solutions…
Radiology
Rad AI Unveils Next-Generation Speech Recognition That Redefines Radiology Reporting
Breakthrough technology delivers faster, more accurate reports through proprietary multi-model AI and advanced language understanding Builds on Rad AI’s broader reporting innovations, including its RSNA Ventures collaboration, to advance the next generation of radiology efficiency…
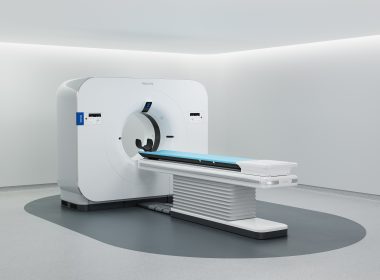
Philips launches Verida, world’s first detector-based spectral CT powered by breakthrough AI, to advance diagnostic precision
Philips Verida spectral CT system, angle view
Philips Verida spectral CT system, angle view
Philips Verida spectral CT system, straight-on view
Philips Verida spectral CT system, straight-on view
November 30, 2025 Philips pioneered detector-based spectral CT, which has been widely adopted in clinical routine exams across anatomies, supported by over 800 peer-reviewed publications [1]CE-marked, 510k pending Verida CT [2] integrates AI across the imaging chain, providing superb image quality while accelerating workflow and reducing dose [3, 4] Amsterdam, the Netherlands and Chicago, USA – At RSNA 2025, Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Verida, the world’s first detector-based spectral CT fully powered by AI. This marks a transformative milestone in CT, with AI optimizing the entire imaging chain – lowering system noise, elevating image quality, and accelerating clinical workflow. With over 800 global installations and supported by over 800 peer-reviewed publications, Philips’ spectral CT uses PACS-native delivery and has been fully embedded into clinical workflow. Spectral CT measures how tissues absorb different x-ray energy levels, enabling differentiation of materials that appear identical on conventional CT. Philips has pioneered detector-based spectral CT, delivering multiple spectral results from a single scan with no tradeoffs in performance or scan time. Now, by integrating AI across the imaging chain, from acquisition to reconstruction, Philips Verida generates industry-leading, superior spectral image quality with minimal noise, in addition to high-definition conventional images. With its full AI capabilities, Verida can achieve dramatic dose reduction [5] without compromising image quality and reduce energy consumption by up to 45% [6]. “The clinical benefits of Verida will fundamentally change my approach to cardiac imaging,” said Prof. Eliseo Vañó Galván, cardiovascular radiologist, Chairman of the CT & MR Department at Hospital Nuestra. Sra. Del Rosario, Madrid, Spain. “With more comprehensive insights in every cardiac CT, I plan to make spectral imaging routine for all patients – building toward a fully spectral CT department. We evaluated many systems, including photon-counting CT, but chose Philips because it delivers the precision we need in a streamlined, easy-to-use platform. The result is greater diagnostic confidence and the potential to reduce the need for invasive angiograms – not just in cardiology, but across other clinical areas as well [7].” Verida reconstructs 145 images per second, so entire exams automatically appear in less than 30 seconds – 2× faster than before and enabling up to 270 exams every day [8]. Building on Philips’ proprietary Spectral Precise Image technology – a deep learning AI reconstruction engine combined with advanced spectral imaging – and its third-generation Nano-panel Precise dual-layer detector with intrinsic noise reduction optimized for AI, Verida is designed to deliver faster, more dose-efficient spectral reconstructions. This enables clinicians to access rich spectral information from a single scan. “Combining the latest advances in our proven spectral CT technology with AI, our flagship Verida CT system is designed to set a new standard in superior image quality and accelerated scans which are fully embedded in the radiology workflow, all to help clinicians detect and characterize disease earlier, reduce variability in diagnoses, and support efficient treatment pathways – in a single scan,” said Dan Xu, Business Leader of CT at Philips. “While photon-counting CT adds complexity, is yet to move from the research arena into clinical practice, Philips spectral CT has been a clinical workhorse for more than a decade and delivers comparable or better clinical outcomes, standing up to the most demanding throughput and at significantly lower total cost of ownership”. Verida extends Philips’ software-defined CT approach, pairing AI-driven spectral precision to advance both clinical and operational outcomes. Built for high-demand environments, it streamlines workflows, reduces repeat scans, and delivers consistently sharp imaging across all care pathways. Philips is debuting Verida at RSNA 2025, with availability in select markets beginning in 2026 [9]. [1] Data on file. [2] CE marked and 510(k) pending. Not currently available for sale in the US.[3] Andersen MB et al. Impact of spectral body imaging in patients suspected for occult cancer: a prospective study of 503 patients. Eur Radiol2020. doi.org/10.1007/s00330-020-06878-7[4] Andersen MB et al. Economic impact of spectral body imaging in the diagnosis of patients suspected of occult cancer. Insights into Imaging 2021. doi.org/10.1186/s13244-021-01116-0. Results of customer testimonies are not predictive of results in other cases, where results may vary.[5] Dose reduction assessments were performed using reference body protocol. In clinical practice, the use of Spectral Precise Image may reduce CT patient dose depending on the clinical task, patient size, and anatomical location. A consultation with a radiologist and a physicist should be made to determine the appropriate dose to obtain diagnostic image quality for the particular clinical task. [6] Based on Axial Body 3D Scan with 80% dose reduction. Energy savings for system preparation is not included.[7] Spectral CT Verida Premium up to 270 (4 CIRS config) exams a day (16 hours dual shift working day) meeting the needs of radiology departments with extended work hours and very high patient throughput.[8] The statements of the clinician reflect independent opinion and are not intended to imply product performance prior to regulatory clearance.[9] Pending regulatory clearance. For further information, please contact: Anna HogrebePhilips Global External RelationsTel.: +1 416 270 6757E-mail: anna.hogrebe@philips.com About Royal Philips Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home.Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2024 sales of EUR 18 billion and employs approximately 67,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachments
Philips Verida spectral CT system, angle view
Philips Verida spectral CT system, straight-on view
Validation study for EchoSolv HF completed at the Mayo Clinic – Study delivers exceptional results
SYDNEY, Nov. 24, 2025 (GLOBE NEWSWIRE) — AI and Medical Technology Company Echo IQ Limited (ASX: EIQ) (“Echo IQ” or “the Company”) is pleased to advise that it has completed its clinical validation for its heart failure clinical decision support software (“EchoSolv HF”) in collaboration with the Mayo Clinic Platform (“MCP”), a division of the Mayo Clinic, a top ranked US hospital. The MCP Validate program is a unique in-market AI evaluation program which generates an independent and objective report on accuracy, efficacy and susceptibility to bias for AI-based decision software (refer ASX Announcement 1 July 2025).
Sectra at RSNA 2025: Supporting subspecialty workflows and photon-counting CT at the workstation to reduce burnout risk and improve patient care
LINKÖPING, Sweden, Nov. 24, 2025 /PRNewswire/ — At this year’s Radiological Society of North America’s annual meeting (RSNA), international medical imaging IT and cybersecurity company Sectra (STO: SECT B) will present new developments designed to make radiology workflows even more…
GE HealthCare to acquire Intelerad, advancing cloud-enabled enterprise imaging across care settings
Creates a fully-connected, cloud-first imaging ecosystem spanning high-growth outpatient and ambulatory, teleradiology and hospital settings Brings AI and workflow orchestration, cloud PACS, and image sharing, all in a SaaS business model, to GE HealthCare Offers attractive return profile given high growth, recurring revenue base and strong margin profile of Intelerad, […]
Hyland Showcases Enterprise Imaging Innovation at RSNA 2025: Empowering Personalized, Connected Care
From radiology and cardiology to digital pathology and multi-specialty imaging, Hyland enables a single, secure, and interoperable imaging record that enhances clinical decision-making CLEVELAND , Nov. 20, 2025 /PRNewswire/ — Hyland, the pioneer of the Content Innovation Cloud™, will be…
Qure.ai partners with Microsoft to increase access to its lung cancer detection and management suite in the U.S.
Accelerating early detection, reducing clinician burden, and enhancing patient outcomes New York, November 18 2025: Qure.ai, a TIME100 most influential company and global leader in digital healthcare, today announced its collaboration with Microsoft. Qure.ai will onboard its end-to-end lung cancer detection, measurement and management suite of AI-powered solutions onto […]
Spryte Medical Receives FDA IDE Approval to Initiate “INSYTE” US Pivotal Trial evaluating the nOCT Imaging System during Brain Aneurysm Treatment
BEDFORD, Mass., Nov. 19, 2025 (GLOBE NEWSWIRE) — Spryte Medical, a pioneer in intravascular neuro imaging, announced it has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to initiate the INSYTE pivotal trial. The trial will evaluate the safety and effectiveness of Spryte’s neuro Optical Coherence Tomography (nOCT) imaging system for use during intracranial aneurysm treatment procedures.
Delcath Systems Announces Publication of Expert Narrative Review on Percutaneous Hepatic Perfusion for Liver Metastases from Uveal Melanoma
QUEENSBURY, N.Y.–(BUSINESS WIRE)–Delcath Systems, Inc. (Nasdaq: DCTH), (“Delcath” or the “Company”) an interventional oncology company focused on the treatment of primary and metastatic liver cancers, today announced the publication of a narrative review by leading interventional radiologists and oncologists from multiple institutions. The review, titled “Treatment of Liver Metastases from […]