BELLEVUE, Wash.–(BUSINESS WIRE)–Aortica Corporation today announced that FDA has approved a supplement to an ongoing Physician Sponsored IDE study at the University of Washington (UW) sponsored by Principal Investigator & Chief of Vascular Surgery Dr. Benjamin Starnes. The positive decision allows the use of Medtronic’s Valiant NAVION™ stent graft system […]
Regulatory
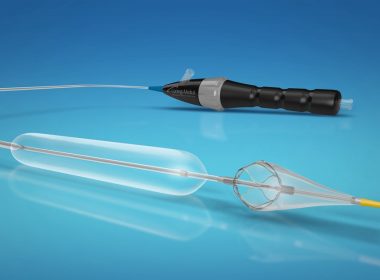
Contego Medical Receives 510(k) Clearance for the Vanguard IEP Peripheral Angioplasty System with Integrated Embolic Protection
RALEIGH, N.C., Dec. 7, 2018 /PRNewswire/ — Contego Medical announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Vanguard IEP® Peripheral Balloon Angioplasty System with Integrated Embolic Protection. Contego Medical is a medical device company developing and commercializing a suite of next-generation devices that address […]
JenaValve Technology Receives FDA Approval for Expanded IDE Enrollment in the Treatment of Patients with Severe Aortic Stenosis and Severe Aortic Regurgitation
IRVINE, Calif.–(BUSINESS WIRE)–JenaValve Technology, Inc., a developer and manufacturer of differentiated transcatheter aortic valve replacement (TAVR) systems, today announced U.S. Food and Drug Administration (FDA) approval of expansion of its Investigational Device Exemption (IDE) feasibility studies for the JenaValve Pericardial TAVR System with the Everdur™ transcatheter heart valve (THV) and […]
Cardinal Health Announces FDA Approval of the INCRAFT® AAA Stent Graft System
DUBLIN, Ohio, November 28, 2018 — Cordis, a Cardinal Health company (NYSE: CAH), today announced that the U.S. Food and Drug Administration (FDA) has approved its INCRAFT® AAA Stent Graft System for use in complex access anatomies. The INCRAFT system is an ultra-low profile and flexible endovascular aneurysm repair (EVAR) system designed […]
MERIT MEDICAL RECEIVES 510(K) APPROVAL FOR EMBOCUBE EMBOLIZATION GELATIN FOR EMBOLIZATION OF HYPERVASCULAR TUMORS
SOUTH JORDAN, Utah – November 29, 2018 – Merit Medical Systems, Inc. (NASDAQ:MMSI), a leading manufacturer and marketer of proprietary disposable devices used primarily in interventional, diagnostic and therapeutic procedures, announces that it has received 510(k) clearance for EmboCube Embolization Gelatin. EmboCube is indicated for use in embolization of hypervascular tumors. The […]
Edwards’ SAPIEN 3 Ultra Transcatheter Heart Valve Receives CE Mark
IRVINE, Calif., Nov. 16, 2018 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced it has received CE Mark for the SAPIEN 3 Ultra system for transcatheter aortic valve replacement in severe, symptomatic aortic stenosis patients. “The SAPIEN 3 Ultra […]
Terumo Cardiovascular Group Announces 510(k) Clearance for the CDI® Blood Parameter Monitoring System 550, Adding New Parameter for Oxygen Delivery
ANN ARBOR, Mich., Nov. 14, 2018 /PRNewswire/ — Terumo Cardiovascular Group, a global leader in cardiovascular surgery technologies, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the CDI® Blood Parameter Monitoring System 550, adding real-time monitoring of oxygen delivery as one of 12 critical blood parameters. Oxygen […]
Medtronic Receives CE Mark Approval for the Valiant Navion(TM) Thoracic Stent Graft System
DUBLIN – November 13, 2018 – Medtronic plc (NYSE:MDT) today announced it has received CE Mark approval for the Valiant Navion(TM) thoracic stent graft system for the minimally invasive repair of all lesions of the descending thoracic aorta, including thoracic aortic aneurysms (TAA), blunt traumatic aortic injuries (BTAI), penetrating atherosclerotic ulcers (PAU), […]
MEDTRONIC BEGINS RENAL DENERVATION STUDY FOR HIGH BLOOD PRESSURE PATIENTS PRESCRIBED ANTI-HYPERTENSIVE MEDICATION
DUBLIN – November 8, 2018 – Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) approval to begin a clinical trial to evaluate the Symplicity Spyral(TM) renal denervation system in patients with high blood pressure (hypertension) who are already prescribed anti-hypertension medications. The SPYRAL HTN-ON MED Trial is the […]
Micro Medical Solutions’ New MicroStent with 120 cm Delivery Catheter Receives CE Mark Approval
WILMINGTON, Mass., Nov. 5, 2018 /PRNewswire/ — Micro Medical Solutions (MMS), an innovator in the field of microvascular interventions designed to improve clinical outcomes and quality of life, announced today that it has received CE Mark approval for a 4Fr.-compatible MicroStent 120 cm delivery catheter, as well as MicroStent devices in five […]