KFAR-SABA, Israel–(BUSINESS WIRE)–CathWorks announced that it has submitted its CathWorks FFRangio™ System to the United States Food & Drug Administration (FDA) for review and 510(k) market clearance. The CathWorks FFRangio System is the non-invasive FFR platform that quickly and precisely delivers objective multi-vessel physiologic measurements to cost-effectively optimize and confirm intraprocedural PCI […]
Regulatory
GORE® Molding & Occlusion Balloon for Endovascular Aortic Repair Receives Approval in the United States, Japan, and Europe
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) announced FDA 510(k) clearance, approval from the Japanese Ministry of Health, Labour, and Welfare, and receipt of CE Mark for the innovative GORE® Molding & Occlusion Balloon, a compliant polyurethane balloon catheter designed in close collaboration with clinicians to assist in the expansion […]
BioSig Technologies Announces FDA 510(k) Clearance for PURE EP System
Santa Monica, CA, Aug. 14, 2018 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (OTCQB: BSGM), announced that the Company has received 510(k) clearance for its first product, PURE EP System, from the U.S. Food and Drug Administration (FDA). The non-invasive PURE EP System is a computerized system intended for acquiring, digitizing, amplifying, filtering, measuring […]
Medicure Announces FDA Approval of Acute Care Cardiovascular Drug Sodium Nitroprusside Injection
WINNIPEG, Aug. 13, 2018 /PRNewswire/ – Medicure Inc. (“Medicure” or the “Company”) (TSXV:MPH, OTC:MCUJF), a cardiovascular pharmaceutical company, is pleased to announce that the United States Food and Drug Administration (“FDA”) has approved its Abbreviated New Drug Application (“ANDA”) for Sodium Nitroprusside Injection 50 mg/2 mL (25 mg/mL) single dose vial (“SNP”), […]
JenaValve Technology Initiates U.S. Patient Enrollment in Early Feasibility Study of Next-Generation TAVR System for the Treatment of Severe Aortic Stenosis and Severe Aortic Regurgitation
IRVINE, Calif.–(BUSINESS WIRE)–JenaValve Technology, Inc., a developer and manufacturer of differentiated transcatheter aortic valve replacement (TAVR) systems, today announced initiation of patient enrollment in the Early Feasibility Study (EFS) of its next generation JenaValve Pericardial TAVR System using the Everdur™ transcatheter heart valve (THV) and CoronatixTMTransfemoral Delivery Catheter at NewYork-Presbyterian/ […]
Thermedical Announces FDA Investigational Device Exemption Approval to Begin US Clinical Study of Durablate Catheter for Treatment of Ventricular Tachycardia
WALTHAM, Mass.–(BUSINESS WIRE)–Thermedical® announced today that the U.S. Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application for the Early Feasibility Study (EFS) of the groundbreaking Durablate™ ablation catheter. The single-arm, observational study is designed to evaluate the safety and effectiveness of the Durablate catheter to […]
Integer Receives FDA Approval for Thin Wall Radial Introducer Kit, RadialSeal™
PLYMOUTH, Minn., July 31, 2018 (GLOBE NEWSWIRE) — Integer Holdings Corporation (“Integer”) (NYSE:ITGR), a leading medical device outsource manufacturer, announced today that it has received FDA and CE Mark approval for their new radial access introducer, RadialSeal™. The RadialSeal Introducer Kit is founded on Integer’s core technology platforms in guidewires and […]
Medtronic Receives FDA Approval for Implantable System for Remodulin® to Treat Patients with Pulmonary Arterial Hypertension
DUBLIN – July 31, 2018 – Medtronic plc (NYSE:MDT) has received U.S. Food and Drug Administration (FDA) approval for the Implantable System for Remodulin® (ISR) to treat patients with pulmonary arterial hypertension (PAH). Through a first-of-its-kind collaboration, the Medtronic SynchroMed(TM) II drug delivery system and cardiac catheter technologies were leveraged to […]
LifeSignals Announces FDA Clearance of Health Care Vital Sign Wireless Monitoring Patch and ECG Application
FREMONT, Calif.–(BUSINESS WIRE)–LifeSignals announced today that it received FDA clearance for its wireless LP1100 Life Signal Patch for enabling the next generation of wearable, healthcare monitoring devices. It is built on two solid technology foundations to provide unprecedented attributes unachieved by another ECG patch product to date. It deploys the […]
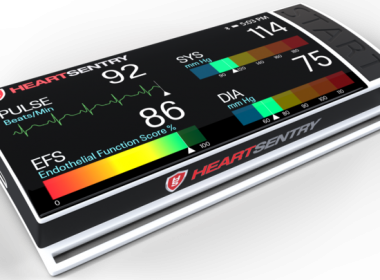
Lexington Biosciences Receives HeartSentry USPTO Patent Approval
VANCOUVER, British Columbia, July 25, 2018 (GLOBE NEWSWIRE) — Lexington Biosciences, Inc. (CSE:LNB) (OTCQB:LXGTF) (the “Company” or “Lexington”), a development-stage medical device company, is pleased to announce the issuance of U.S. Patent No. 10,028,664, which covers aspects of its HeartSentry device. “Today’s news is a big deal for Lexington,” says […]