SHEFAYIM, Israel–(BUSINESS WIRE)–Zebra Medical Vision (http://zebra-med.com/) announces today it has received 510(k) clearance for its Coronary Calcium Scoring algorithm. The algorithm, capable of automatically calculating a patient’s Agatston equivalent coronary calcium score from ECG gated CT scan, provides physicians with important data used in the assessment of the risk for coronary […]
Regulatory
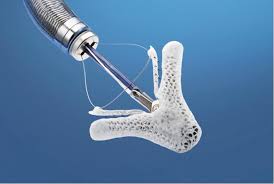
Abbott Receives FDA Approval for Next-Generation MitraClip® Device to Treat People with Leaky Heart Valves
ABBOTT PARK, Ill., July 12, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced it received approval from the U.S. Food and Drug Administration (FDA) for a next-generation version of its leading MitraClip® heart valve repair device used to repair a leaky mitral valve without open-heart surgery. The transcatheter clip-based therapy, now on a third generation […]
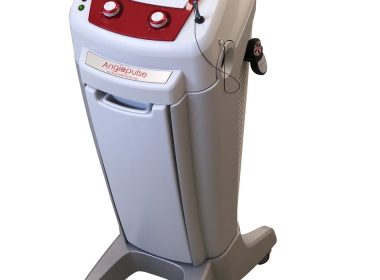
Angiodroid Srl Announces CE Mark for ANGIOPULSE™ Intra-aortic Balloon Pump
BOLOGNA, Italy, July 11, 2018 /PRNewswire/ — ANGIODROID Srl, an Italian manufacturer of medical technologies, announces the CE mark for ANGIOPULSE, its first intra-aortic balloon pump (IABP) device. The new ANGIOPULSE IABP incorporates a new system for IABP management based on pressure regulation. Extremely sophisticated, ANGIOPULSE builds on the Italian experience of Angiodroid Srl (angiodroid.com), […]
Medtronic Receives FDA Approval for Less-Invasive Heart Pump Implant Procedure
DUBLIN – July 11, 2018 – Medtronic plc (NYSE: MDT) has received United States Food and Drug Administration (FDA) approval for a less-invasive implant approach of its HVAD(TM) System, a left ventricular assist device (LVAD) for patients with advanced heart failure. The HVAD System is the smallest commercially available LVAD, and […]
ReCor Medical Announces FDA Approval Of IDE For Pivotal Study Of Paradise® Ultrasound Denervation System For Treatment Of Hypertension
PALO ALTO, Calif., July 2, 2018 /PRNewswire/ — ReCor Medical announced today that the US Food & Drug Administration approved the Company’s new pivotal study of the Paradise Ultrasound Denervation System for the treatment of hypertension: RADIANCE-II. Building upon the recent positive results of the Company’s RADIANCE-HTN SOLO study, RADIANCE-II will be […]
Embolx Announces FDA Clearance of Next Generation Sniper Balloon Occlusion Microcatheter to Deliver Pressure-Directed Therapy for Tumors, Enlarged Prostate and Fibroids
SUNNYVALE, Calif.–(BUSINESS WIRE)–Embolx, Inc., a medical device company developing microcatheters for arterial embolization procedures, today announced that the company has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its next generation family of Sniper® Balloon Occlusion Microcatheters, an innovative system for pressure-directed arterial embolization therapy. […]
Beckman Coulter Diagnostics Receives U.S. FDA 510(k) Clearance for High- sensitivity Access hsTnI Assay
BREA, Calif., June 27, 2018 /PRNewswire/ — Beckman Coulter Diagnostics announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new high-sensitivity troponin (hsTnI) assay, Access hsTnI, for use on the Access 2, DxI and the entire Access family of immunoassay systems. The Access hsTnI assay […]
Avenu Medical Receives FDA Approval for Ellipsys Vascular Access System for Non-Surgical Dialysis Fistula Creation
SAN JUAN CAPISTRANO, Calif.–(BUSINESS WIRE)–Avenu Medical, Inc. announced today that it has received De Novo marketing authorization from the Food and Drug Administration (FDA) to market its Ellipsys® Vascular Access System, an innovative, minimally-invasive catheter-based system designed for End-Stage Renal Disease (ESRD) patients requiring hemodialysis. The FDA’s action will provide U.S. […]
Shape Memory Medical Receives FDA Clearance for the IMPEDE® Embolization Plug
SANTA CLARA, Calif.–(BUSINESS WIRE)–Shape Memory Medical Inc. announced today it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the IMPEDE Embolization Plug. The IMPEDE Embolization Plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. It is available in […]
Caladrius Receives FDA Regenerative Medicine Advanced Therapy Designation for CD34+ Cell Therapy for Treating Refractory Angina
BASKING RIDGE, N.J., June 19, 2018 (GLOBE NEWSWIRE) — Caladrius Biosciences, Inc. (Nasdaq:CLBS) (“Caladrius” or the “Company”), a clinical-stage biopharmaceutical company with multiple technology platforms targeting select cardiovascular indications and autoimmune diseases, announces today that the U.S. Food and Drug Administration (“FDA”) has granted regenerative medicine advanced therapy (“RMAT”) designation to […]