Clinical evidence demonstrates that AtriClip® devices exclude and electrically isolate the left atrial appendage MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in surgical treatment for atrial fibrillation (Afib) and left atrial appendage (LAA) management, today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance of […]
Rhythm
First-ever: FDA Clears Biobeat’s Wearable Watch and Patch for Non-invasive Cuffless Monitoring of Blood Pressure
TEL AVIV, Israel, Aug. 26, 2019 /PRNewswire/ — Biobeat, a bio-medical technology company developing advanced sensing and remote monitoring solutions for patients, announced today that the U.S. Food and Drug Administration (FDA) has granted a 510K clearance for its patch and watch for measurement of blood pressure, oxygenation and heart rate in hospitals, clinics, long-term […]
Adagio Medical Announces US FDA Investigational Device Exemption Approval With Conditions To Conduct A Clinical Study For Treatment Of Persistent Atrial Fibrillation Using The Ultra-Low Temperature Cryoablation System
LAGUNA HILLS, Calif., Aug. 19, 2019 /PRNewswire/ — Adagio Medical, Inc. (Adagio), the developer of iCLAS™, the company’s ultra-low temperature intelligent continuous lesion ablation system, announced that it has received an Investigational Device Exemption approval with conditions from the US Food and Drug Administration (FDA) to conduct a non-randomized, single-arm clinical study for persistent atrial […]
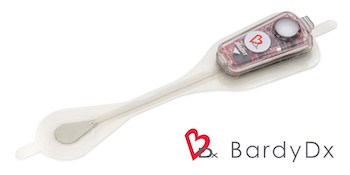
Bardy Diagnostics™ Selected As Finalist For Two MedTech Insight Awards To Be Announced At AdvaMed’s Annual MedTech Conference
SEATTLE, Aug. 19, 2019 /PRNewswire/ — Bardy Diagnostics, Inc., (“BardyDx”), a leading provider of ambulatory cardiac monitoring technologies and custom data solutions, announced that Informa Pharma Intelligence, a leading international healthcare intelligence consultancy, has recognized BardyDx as a finalist in two categories for the 2nd Annual Medtech Insight Awards for “Best Technical Innovation: Diagnostics” and “Best Proof-of-Value of an […]
Philips unveils HeartStart Intrepid with IntelliSpace Connect across Europe and select markets worldwide
Enhanced communication and seamless data sharing capabilities augment clinical and operational efficiencies for patient assessment across emergency care settings Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of the HeartStart Intrepid monitor/defibrillator with IntelliSpace Connect. Created to provide clinical and operational […]
FDA approves new device to improve symptoms in patients with advanced heart failure
SILVER SPRING, Md., Aug. 16, 2019 /PRNewswire/ — The U.S. Food and Drug Administration today approved the Barostim Neo System for the improvement of symptoms in patients with advanced heart failure who are not suited for treatment with other heart failure devices, such as cardiac resynchronization therapy. The FDA gave the device […]
DynoSense Corp. Announces FDA Approval of Its Cloud Based Vital Signs Measurement System
SAN JOSE, Calif., Aug. 13, 2019 /PRNewswire/ — DynoSense Corp. announced today that the U.S. Food and Drug Administration (FDA) has granted the company clearance for its patented Vital Signs Measuring System, the world’s first most integrated and cloud-based vital signs measuring and recording platform. Its unique worldwide patented and award-winning design is as simple […]
Bardy Diagnostics™ Selected as One of Six Disruptive MedTech Startups For the HealthTech Arkansas Accelerator Program
SEATTLE, Aug. 9, 2019 /PRNewswire/ — Bardy Diagnostics, Inc., (“BardyDx”), a leading provider of ambulatory cardiac monitoring technologies and custom data solutions, announced that HealthTech Arkansas, a healthcare accelerator and investment fund that connects early-stage healthcare companies with disruptive technologies to Arkansas healthcare providers, has selected BardyDx to participate in the organization’s 2019 accelerator program. BardyDx […]
ScottCare and UTHealth EP Heart Program Partner to Educate the Next Wave of Cardiac Implantable Electronic Device Specialists
CLEVELAND & HOUSTON–(BUSINESS WIRE)–ScottCare Cardiovascular Solutions and the EP Heart Cardiovascular Electrophysiology Training Program at The University of Texas Health Science Center at Houston (UTHealth) today announced a collaboration intended to provide an intensive training experience for individuals interested in pursuing a career as Cardiac Implantable Electronic Device Specialists and […]
BioSig Allowed Additional Foundational US Patent for its PURE EP(tm) System
Westport, CT, Aug. 01, 2019 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (NASDAQ: BSGM), a medical device company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the electrophysiology (EP) marketplace, today announced that the US Patent Office has allowed another foundational patent including additional 29 patent claims […]