AUSTIN, Texas, Jan. 18, 2018 /PRNewswire/ — On Nov. 14, 2017, Texas Cardiac Arrhythmia Institute (TCAI) at St. David’s Medical Center became the first hospital in Texas—and among the first in the nation—to use the new smartphone compatible Confirm Rx™ Insertable Cardiac Monitor (ICM), the world’s first and only smartphone compatible ICM designed to help physicians […]
Rhythm
FDA adds safety alert for Zoll LifeVest
Patient death prompts safety warning by FDA. Zoll LifeVest 4000 Wearable Cardioverter Defibrillator: FDA Safety Communication – Potential Lack of Treatment (Shock) Delivery Due to Device Failure by FDA.gov ISSUE: FDA is providing information and recommendations regarding the Zoll LifeVest 4000 due to concerns that the device may fail to […]
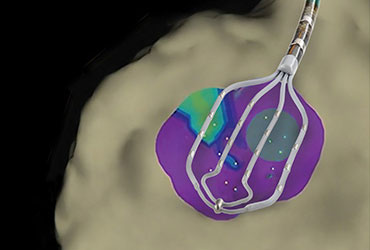
Abbott announces European launch of Advisor HD Grid Mapping Catheter, Sensor Enabled
ABBOTT PARK, Ill., Jan. 11, 2018 – Abbott today announced CE Mark approval for the company’s new Advisor™ HD Grid Mapping Catheter, Sensor Enabled™, a product designed to advance cardiac mapping during cardiac ablation to treat patients with complex cardiac arrhythmias. With the European launch of this latest addition to […]
CorInnova Wins Top Prize at “RESI San Francisco 2018” Innovation Challenge
HOUSTON–(BUSINESS WIRE)–CorInnova Inc., an emerging medical device company developing breakthrough technology for the treatment of Heart Failure, announced today that it has won First Prize in the “RESI (Redefining Early Stage Investments) San Francisco 2018” investment competition for early-stage life science companies. “We are excited that early-stage investors instantly grasp the […]
iMedrix Announces General Availability of KardioScreen, a CE certified, Connected ECG Device for Universal Use
BANGALORE, January 5, 2018 /PRNewswire/ — iMedrix, a Silicon Valley and Bangalore-based mHealth start-up, announced the general availability of its product KardioScreen, a CE certified mobile/portable hospital grade digital ECG. KardioScreen is the first truly mobile, connected ECG solution that can be applied universally: field screening, point of care, ambulance, Cardiac ICU and […]
First Patient Enrolled in U.S. IDE Study To Evaluate the Potential of New Device To Reduce Stroke Risk in Atrial Fibrillation Patients
IRVINE, Calif., Jan. 11, 2018 /PRNewswire/ — Johnson & Johnson Medical Devices Companies* today announced that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, enrolled the first patient in the WaveCrest® Investigational Device Exemption (IDE) Trial. The study will evaluate the safety and effectiveness of the WaveCrest® Left Atrial […]
CardioFocus Announces Expanded Partnership With Japan Lifeline
MARLBOROUGH, Mass., Jan. 9, 2018 /PRNewswire/ — CardioFocus Inc., a medical device innovator and manufacturer dedicated to advancing ablation treatment for cardiac disorders such as atrial fibrillation (AF), today announced that it has entered into an expanded partnership with Japan Lifeline. The expanded partnership solidifies the commercial strategy for bringing the HeartLight® Endoscopic […]
Endotronix Announces Successful First-in-Human Implantation of the Cordella Pulmonary Artery Pressure Sensor and Initiation of the SIRONA Clinical Trial
LISLE, Ill., Jan. 8, 2018 /PRNewswire/ — Endotronix, Inc., a medical technology company providing device and digital health solutions for heart failure management, today announced successful first-in-human implantation of the Cordella™ Pulmonary Artery (PA) Pressure Sensor and initiation of the SIRONA First-in-Human (FIH) clinical trial. Cardiologists Prof. Dr. Wilfried Mullens of Ziekenhuis Oost-Limburg and University Hasselt and […]
Sensifree Reports Statistically Significant Concordance of Arterial Pressure Signal Waveform Between its Continuous Non-invasive Blood Pressure Monitoring Technology and Invasive Arterial Line
CUPERTINO, Calif.–(BUSINESS WIRE)– Sensifree, a medical device company developing advanced RF-based hemodynamic monitoring solutions, announces results of a clinical study showing excellent concordance between the signal morphology of its RF based sensor and invasive arterial line, the standard of care for continuous blood pressure monitoring. Clinical data from the study further […]
BioSig Technologies Appoints New CFO to Facilitate Growth Trajectory
Santa Monica, CA, Jan. 03, 2018 (GLOBE NEWSWIRE) — BioSig Technologies(OTCQB: BSGM), a medical device company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the $4.6 billion electrophysiology (EP) marketplace, today announced that Mr. Steven (Steve) Chaussy will be joining the company to serve in […]