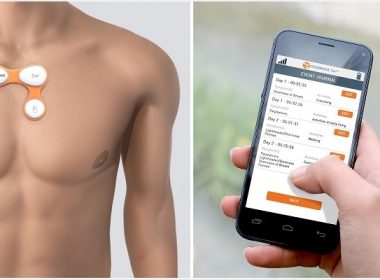
ABBOTT PARK, Ill., Oct. 23, 2017 /PRNewswire/ — Abbott (NYSE: ABT) has secured U.S. Food and Drug Administration (FDA) clearance for the Confirm Rx™ Insertable Cardiac Monitor (ICM), the world’s first and only smartphone compatible ICM designed to help physicians remotely identify cardiac arrhythmias. With FDA clearance Abbott can now provide U.S. patients a new […]
Rhythm
BioSig Technologies Advances Towards Commercialization into the Global Electrophysiology Market Estimated to Reach $8.2 Billion by 2022
Minneapolis, MN, Oct. 19, 2017 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (BSGM), a medical device company developing a proprietary platform designed to address an unmet technology need within the emerging electrophysiology (EP) marketplace, announced today that they have made significant progress towards commercialization of their proprietary PURE EP System. The […]
HeartSciences Launches Breakthrough ECG Technology at the Canadian Cardiovascular Congress
MyoVista® hsECG™ Utilizes Wavelet Technology to Detect Cardiac Diastolic Dysfunction SOUTHLAKE, Texas, Oct. 19, 2017 /PRNewswire-USNewswire/ — HeartSciences announced today that the company will launch its MyoVista® high sensitivity electrocardiograph (hsECG™) Testing Device in Canada at the Canadian Cardiovascular Congress (CCC) in Vancouver on October 21-24. The MyoVista hsECG is now available in Canada, where it received Health Canada Medical […]
FDA Grants Marketing Clearance for the Peerbridge Cor™ Multi-channel Remote ECG Monitor
NEW YORK, Oct. 4, 2017 /PRNewswire/ — Peerbridge Health Inc., a Health IT company, announced today that its first product, the Peerbridge Cor™ System — a wireless electrocardiogram (ECG) monitor — has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The patented Peerbridge Cor has the smallest on-body footprint of any […]
Cardiologs Raises $6.4 Million to Lead the AI Revolution in Cardiology
PARIS & SAN FRANCISCO–(BUSINESS WIRE)–Cardiologs Technologies announced today that it has raised $6.4 million in a Series A financing with a syndicate of life science and technology investors (Idinvest, ISAI, Kurma Partners, Partech Ventures) and with continued support and participation from current investor Bpifrance seed fund (F3A). This round brings total […]
BIOTRONIK CLS Technology Shown To Reduce Fainting In Syncope Patients By Seven-Fold
Robustly Positive Results of SPAIN Study Published in Journal of American College of Cardiology BERLIN, Germany, October 4, 2017 – BIOTRONIK’s exclusive rate responsive technology, Closed Loop Stimulation (CLS) has shown to reduce fainting in pacemaker patients with vasovagal syncope up to sevenfold. Results from the SPAIN study, the first […]
Boston Scientific (BSX) to Acquire SoCal’s Apama Medical in $300 Million Deal
MARLBOROUGH, Mass., Oct. 2, 2017 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced a definitive agreement to acquire Apama Medical Inc., a privately-held company that is developing the Apama Radiofrequency (RF) Balloon Catheter System for the treatment of atrial fibrillation (AF). The transaction consists of $175 million in cash up-front and a maximum […]
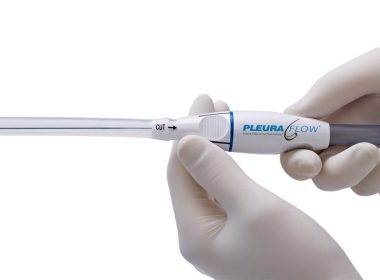
Newly Published Peer Reviewed Study Reveals Benefits Of ClearFlow’s PleuraFlow ACT System
ANAHEIM, Calif.–(BUSINESS WIRE)–ClearFlow Inc., a medical device company based in Anaheim, California, has announced the publication of significantly positive results in a study evaluating the company’s PleuraFlow® Active Clearance Technology® System. Data from a peer-reviewed clinical study indicating a marked reduction in Postoperative Atrial Fibrillation (POAF) among patients with active clearance of chest […]
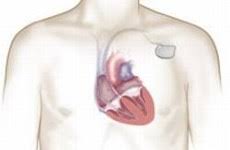
LivaNova (LIVN) And MicroPort Scientific (MCRPF) Announce The Approval Of Rega Pacemakers By The China Food And Drug Administration
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN) (“LivaNova”) and MicroPort Scientific Corporation (HK:00853) (“MicroPort”), today announced that the companies’ Shanghai-based joint venture MicroPort Sorin Cardiac Rhythm Management Co. Ltd. (“MSC” or the “joint venture”) obtained approval for its family of RegaTM pacemakers from the China Food and Drug Administration. Not only are Rega pacemakers […]
CardioFocus® Announces European CE Mark Approval Of The Next-Generation HeartLight® Excalibur Balloon™ Designed For The Treatment Of Atrial Fibrillation
MARLBOROUGH, Mass., Sept. 27, 2017 /PRNewswire/ — CardioFocus, Inc. today announced the European CE Mark approval of the HeartLight Excalibur Balloon, a next-generation technology designed for the treatment of atrial fibrillation (AF). The Excalibur Balloon leverages the proven universal balloon design of the company’s FDA-approved HeartLight® Endoscopic Ablation System and introduces an advanced feature set that optimizes the speed […]