MARLBOROUGH, Mass., Sept. 25, 2017 /PRNewswire/ — Boston Scientific (NYSE: BSX) has launched the Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) systems featuring the HeartLogic Heart Failure Diagnostic to help physicians improve heart failure (HF) management. The new devices, which are approved by the U.S. Food […]
Rhythm
FDA OKs Abbott (ABT)’ MRI-Compatibility for the Company’s Ellipse ICD
ABBOTT PARK, Ill., Sept. 22, 2017 /PRNewswire/ — Abbott (NYSE: ABT) today announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labeling for one of Abbott’s most widely-used implantable cardioverter defibrillators (ICD) and associated high voltage leads. The approval of MR-conditional labeling for the Ellipse ICD with the Tendril MRI […]
Boston Scientific (BSX) Announces Positive Late-Breaking Clinical Trial Data For The Heartlogic Heart Failure Diagnostic
DALLAS and MARLBOROUGH, Mass., Sept. 19, 2017 /PRNewswire/ — Boston Scientific (NYSE: BSX) today announced new data from the Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients (MultiSENSE) study evaluating the performance of the HeartLogic Heart Failure Diagnostic to predict impending heart failure (HF) decompensation. Data from the study were presented as a late-breaking clinical […]
Cardihab Cardiac Rehab App Spun Out Of CSIRO Ehealth Division
BRISBANE, Australia–(BUSINESS WIRE)–An Australian solution to dramatically improve recovery from a heart attack today became reality. Cardihab’s technology platform was developed by scientists at the Australian eHealth Research Centre (AEHRC), a joint venture between CSIRO and the Queensland Government. Cardihab was spun-out from CSIRO after raising venture capital investment of […]
2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction
The 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction, developed in collaboration with the ACC, AHA, APHRS, ASA, EHRA, IDSA, LAHRS, PACES, and STS, is intended to help clinicians in their decision-making process for managing leads and builds on the 2009 Transvenous Lead Extraction: Heart Rhythm Society Expert […]
How Boston Scientific (BSX) Is Boosting Medical Device Value
Boston Scientific over the summer unveiled a new value-add for its implantable cardio devices: an online Trugevity calculator that lets physicians predict battery life. The idea is to help health providers quickly grasp the benefits of Boston Sci’s longer lasting EnduraLife battery technology, which the company touts has nine clinical […]
LivaNova (LIVN) Considers Selling Its $250 Million Cardiac Rhythm Management Biz
September 14, 2017: LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN) (“LivaNova” or the “Company”), a market-leading medical technology and innovation company, today announced it is exploring strategic options for its Cardiac Rhythm Management (“CRM”) Business Franchise. “As part of our ongoing efforts to position LivaNova for long-term success, we are sharpening our focus […]
AtriCure Announces U.S. Launch of the AtriClip® PRO•V™ Device
MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in treatments for atrial fibrillation (Afib) and left atrial appendage management, today announced that it has launched the AtriClip PRO•V™ Left Atrial Appendage (LAA) Exclusion System in the United States. The new device offers an open-ended design combined with a tip-first closure […]
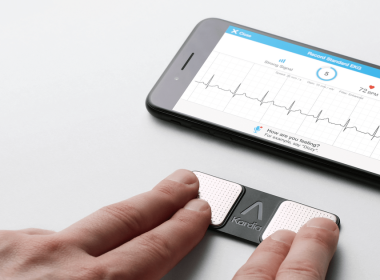
New Research Confirms Significance of AliveCor’s 30 Second EKG
MOUNTAIN VIEW, Calif., Sept. 6, 2017 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (EKG) technology, today announced the results of four clinical research presentations that demonstrate that AliveCor’s hyper-fast, 30 second, digital EKG can positively impact and potentially even save the lives of millions of people around the world who suffer […]
ABBOTT ISSUES NEW UPDATES FOR IMPLANTED CARDIAC DEVICES
ABBOTT PARK, Ill., Aug. 29, 2017 – Today, Abbott notified physicians of updates to its implantable pacemakers and defibrillators as part of its ongoing commitment to continuously improve patient care. The new device updates include a Battery Performance Alert for our implantable cardioverter defibrillators (ICDs) that provides physicians with earlier […]