Fujifilm’s LISENDO 800 Ultrasound System Connects to AI-driven Us2.ai, providing users with fully automated analyses of all heart chambers using both 2D and doppler views LEXINGTON, Mass.–(BUSINESS WIRE)–FUJIFILM Healthcare Americas Corporation, a leading provider of diagnostic and enterprise imaging solutions and Us2.ai, a leader in AI-automated echocardiography solutions, have partnered to […]
Uncategorized
GE HealthCare expands invasive cardiology solutions portfolio with AltiX AI.i for elevated experience in catheterization lab and electrophysiology procedures
AltiX AI.i edition of Mac-Lab, CardioLab and ComboLab can enhance efficiency and precision care for multiple types of cardiac procedures CHICAGO–(BUSINESS WIRE)–GE HealthCare (Nasdaq: GEHC) today announced the launch of the AltiX AI.i edition of Mac-Lab™, CardioLab™ and ComboLab™. The AltiX AI.i editions are designed to improve the user experience, […]
NewVue.ai and RamSoft Announce Strategic Partnership to Deliver Next-Generation Radiology Workflow Orchestration
LAS VEGAS, March 3, 2025 /PRNewswire/ – NewVue.ai, a leader in AI-driven radiology workflow orchestration, and RamSoft®, a global provider of cloud-based imaging and RIS/PACS solutions, today announced a strategic reseller partnership. RamSoft will integrate and offer NewVue.ai’s…
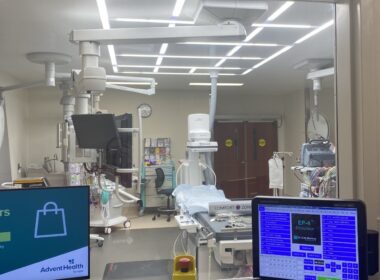
Pepin Heart Institute at AdventHealth Tampa expands with fourth electrophysiology lab
TAMPA, Fla., Feb. 10, 2025 /PRNewswire/ — The AdventHealth Pepin Heart Institute has further strengthened its commitment to providing world-class cardiac care by opening its fourth dedicated Electrophysiology (EP) Lab. This state-of-the-art expansion will allow for even more advanced…
Medera’s Novoheart Expands Human Cardiac Research with CTScreen™ Installation at University Medical Center, Utrecht
Cutting-Edge Cardiac Research: Novoheart’s CTScreen™ system has been installed at UMC Utrecht, equipping Regenerative Medicine Center with advanced high-throughput tools to accelerate human cardiac disease modeling and therapeutic screening, including both drug candidates and gene editing approachesDriving Innovation: This installation paves the way for future co-development projects with UMC Utrecht, advancing human-based research and cardiac regenerative technologies BOSTON and UTRECHT, Netherlands, Feb. 10, 2025 (GLOBE NEWSWIRE) — Medera Inc. (“Medera”), a clinical-stage biopharmaceutical company focused on targeting difficult-to-treat or currently incurable diseases with significant unmet needs, and Novoheart, its wholly owned pre-clinical subsidiary pioneering human-based cardiac tissue engineering for disease modelling and drug screening, are pleased to announce the installation of their state-of-the-art CTScreen™ platform at the University Medical Center Utrecht (UMC Utrecht). This milestone equips the Regenerative Medicine Center Utrecht with a leading-edge system to model human cardiac diseases, develop therapies, and transform preclinical cardiac research. UMC Utrecht researchers will leverage CTScreen™ to study disease-specific mechanisms, screen therapeutic candidates, and optimize drug safety using Novoheart’s human-based assays. The CTScreen™ system supports Novoheart’s renowned “mini-Heart™” platform, enabling automated screening of bioengineered human cardiac tissues that accurately mimic heart function. Using a standard 96-well format, the system can evaluate up to 96 tissue samples at a time, allowing early detection of therapeutic effects and significantly accelerating the development process. This installation builds upon Novoheart’s successful collaborations with global leaders like AstraZeneca and Curi Bio, while advancing the goals of the FDA Modernization Act 2.0 through human-based alternatives to traditional methods. “This technology enables unparalleled precision in understanding cardiac conditions,” said Kevin Costa, Chief Scientific Officer and co-founder of Novoheart. “With UMC Utrecht’s expertise in regenerative medicine, we anticipate breakthroughs that will transform cardiovascular research.” “Our team is excited to bring the CTScreen™ system into our facility,” said Joost Sluijter, Ph.D., Director of the Heart and Lung Division within the Regenerative Medicine Center Utrecht. “This adds much-needed capabilities for measuring human cardiac tissue contractility, advancing our efforts in studying disease mechanisms and accelerating translation to clinical trials for patients with familial cardiomyopathies and other heart diseases that lack effective treatments.” “The installation at UMC Utrecht represents not only an expansion of Novoheart’s reach, but also an exciting step in strengthening our partnership with this world-renowned institution,” concluded Ronald Li, Ph.D., Chief Executive Officer and Founder of Medera. “Together, we are exploring innovative possibilities in cardiac regeneration technologies that have the potential to redefine what’s possible in heart disease treatment. While today’s announcement marks a key milestone, we look forward to sharing more exciting developments soon as our collaboration deepens.” On September 5, 2024, Medera and Keen Vision Acquisition Corporation (“KVAC”) (Nasdaq: KVAC, KVACW), announced they had entered into a definitive merger agreement. About Medera Medera (www.medera.bio) is a clinical-stage biopharmaceutical company focused on targeting difficult-to-treat or currently incurable diseases with significant unmet needs, utilizing next-generation gene and cell-based approaches in combination with bioengineered human-based technology (including the mini-Heart platform). Medera operates via the two preclinical and clinical business units, Novoheart and Sardocor, respectively. Novoheart capitalizes on the world’s first and award-winning “mini-Heart” Technology for revolutionary disease modelling and drug discovery, uniquely enabling the modelling of human-specific diseases and discovery of therapeutic candidates free from species-specific differences in accordance to the FDA Modernization Act 2.0. Novoheart’s versatile technology platform provides a range of state-of-the-art automation hardware and software as well as screening services, for human-specific disease modelling, therapeutic target discovery and validation, drug toxicity and efficacy screening, and dosage optimization carried out in the context of healthy and/or diseased human heart chambers and tissues. Global pharmaceutical and academic leaders are using Novoheart’s technology platform for their drug discovery and development purposes. The Novoheart platform has facilitated and accelerated the development of Sardocor’s lead therapeutic candidates that are currently in clinical trials. Sardocor is dedicated to the clinical development of novel next-generation therapies for Medera. Leveraging Novoheart’s human-based drug discovery and validation platforms, Sardocor aims to expedite drug development and regulatory timelines for its gene and cell therapy pipeline. Sardocor has received Investigational New Drug (IND) clearances from the FDA for three ongoing AAV-based cardiac gene therapy clinical trials targeting Heart Failure with Reduced Ejection Fraction (HFrEF), Heart Failure with Preserved Ejection Fraction (HFpEF) with the Fast Track Designation, and Duchenne Muscular Dystrophy-induced Cardiomyopathy (DMD-CM) with the Orphan Drug Designation. Additionally, Sardocor’s pipeline includes four preclinical gene therapy and three preclinical small molecule candidates targeting various cardiac, pulmonary, and vascular diseases. About Keen Vision Acquisition Corporation Keen Vision Acquisition Corp (“KVAC”), listed on Nasdaq, is a blank check company incorporated for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses or entities. KVAC is focused on biotechnology, consumer goods or agriculture opportunities, which are also evaluated on their sustainability, environmental, social, and corporate governance (“ESG”) imperatives. EF Hutton LLC and Brookline Capital Markets, a division of Arcadia Securities, LLC, are serving as Capital Markets Advisors for KVAC. www.kv-ac.com Forward-Looking Statements Certain statements included in this press release are not historical facts but are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this press release are forward-looking statements. Any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are also forward-looking statements. In some cases, you can identify forward-looking statements by words such as “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “strategy,” “future,” “opportunity,” “may,” “target,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” “preliminary,” or similar expressions that predict or indicate future events or trends or that are not statements of historical matters, but the absence of these words does not mean that a statement is not forward-looking. Forward-looking statements include, without limitation, KVAC’s, Medera’s, or their respective management teams’ expectations concerning the outlook for their or Medera’s business, productivity, plans, and goals for future operational improvements and capital investments, operational performance, future market conditions, or economic performance and developments in the capital and credit markets and expected future financial performance, including expected net proceeds, expected additional funding, the percentage of redemptions of KVAC’s public shareholders, growth prospects and outlook of Medera’ operations, individually or in the aggregate, including the achievement of project milestones, commencement and completion of commercial operations of certain of Medera’s projects, as well as any information concerning possible or assumed future results of operations of Medera. Forward-looking statements also include statements regarding the expected benefits of the transactions contemplated by the merger (“Transaction”). The forward-looking statements are based on the current expectations of the respective management teams of Medera and KVAC, as applicable, and are inherently subject to uncertainties and changes in circumstance and their potential effects. There can be no assurance that future developments will be those that have been anticipated. These forward-looking statements involve a number of risks, uncertainties or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, (i) the risk that the Transaction may not be completed in a timely manner or at all, which may adversely affect the price of KVAC’s securities; (ii) the risk that the Transaction may not be completed by KVAC’s business combination deadline and the potential failure to obtain an extension of the business combination deadline if sought by KVAC; (iii) the failure to satisfy the conditions to the consummation of the Transaction, including the adoption of the Merger Agreement by the shareholders of KVAC and the receipt of certain regulatory approvals; (iv) market risks; (v) the occurrence of any event, change or other circumstance that could give rise to the termination of the Merger Agreement; (vi) the effect of the announcement or pendency of the Transaction on Medera’s business relationships, performance, and business generally; (vii) the outcome of any legal proceedings that may be instituted against Medera or KVAC related to the Merger Agreement or the Transaction; (viii) failure to realize the anticipated benefits of the Transaction; (ix) the inability to maintain the listing of KVAC’s securities or to meet listing requirements and maintain the listing of Medera’s securities on Nasdaq; (x) the inability to implement business plans, forecasts, and other expectations after the completion of the Transaction, identify and realize additional opportunities, and manage its growth and expanding operations; (xi) risks related to Medera’s ability to develop, license or acquire new therapeutics; (xii) the risk that Medera will need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; (xiii) the risk of product liability or regulatory lawsuits or proceedings relating to Medera’s business; (xiv) uncertainties inherent in the execution, cost, and completion of preclinical studies and clinical trials; (xv) risks related to regulatory review, and approval and commercial development; (xvi) risks associated with intellectual property protection; (xvii) Medera’s limited operating history and risk that it may never successfully commercialise its products; (xviii) Medera expects to continue to incur significant losses and may never achieve or maintain profitability; and (xix) the risk that additional financing in connection with the Transaction may not be raised on favorable terms. The foregoing list is not exhaustive, and there may be additional risks that neither KVAC nor Medera presently knows or that KVAC and Medera currently believe are immaterial. You should carefully consider the foregoing factors, any other factors discussed in this press release and the other risks and uncertainties described in the “Risk Factors” section of KVAC’s Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the SEC on March 29, 2024, the risks to be described in the registration statement, which will include a preliminary proxy statement/prospectus, and those discussed and identified in filings made with the SEC by KVAC from time to time. Medera and KVAC caution you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth in this press release speak only as of the date of this press release. Neither Medera nor KVAC undertakes any obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs. In the event that any forward-looking statement is updated, no inference should be made that Medera or KVAC will make additional updates with respect to that statement, related matters, or any other forward-looking statements. Any corrections or revisions and other important assumptions and factors that could cause actual results to differ materially from forward-looking statements, including discussions of significant risk factors, may appear, up to the consummation of the Transaction, in KVAC’s public filings with the SEC, and which you are advised to review carefully. Important Information for Investors and Shareholders In connection with the Transaction, KVAC and Medera filed a registration statement with the SEC, which includes a prospectus with respect to the securities to be issued in connection with the Transaction and a proxy statement to be distributed to holders of KVAC’s common shares in connection with KVAC’s solicitation of proxies for the vote by KVAC’s shareholders with respect to the Transaction and other matters to be described in the Registration Statement (the “Proxy Statement”). After the SEC declares the registration statement effective, KVAC plans to mail copies to shareholders of KVAC as of a record date to be established for voting on the Transaction. This press release does not contain all the information that should be considered concerning the Transaction and is not a substitute for the registration statement, Proxy Statement or for any other document that KVAC may file with the SEC. Before making any investment or voting decision, investors and security holders of KVAC are urged to read the registration statement and the Proxy Statement, and any amendments or supplements thereto, as well as all other relevant materials filed or that will be filed with the SEC in connection with the Transaction as they become available because they will contain important information about, Medera, KVAC and the Transaction. Investors and security holders will be able to obtain free copies of the registration statement, the Proxy Statement and all other relevant documents filed or that will be filed with the SEC by KVAC through the website maintained by the SEC at www.sec.gov. In addition, the documents filed by KVAC may be obtained free of charge from KVAC’s website at https://www.kv-ac.com or by directing a request to info@kv-ac.com. The information contained on, or that may be accessed through, the websites referenced in this press release is not incorporated by reference into, and is not a part of, this press release. Participants in the Solicitation KVAC, Medera and their respective directors, executive officers and other members of management and employees may, under the rules of the SEC, be deemed to be participants in the solicitations of proxies in connection with the Transaction. For more information about the names, affiliations and interests of KVAC’s directors and executive officers, please refer to KVAC’s annual report on Form 10-K filed with the SEC on March 29, 2024, which can be found at https://www.sec.gov/ix?doc=/Archives/edgar/data/1889983/000121390024027973/ea0201104-10k_keenvision.htm and registration statement, Proxy Statement and other relevant materials filed with the SEC in connection with the Transaction when they become available. Additional information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, which may, in some cases, be different than those of KVAC’s shareholders generally, will be included in the registration statement and the Proxy Statement and other relevant materials when they are filed with the SEC when they become available. Shareholders, potential investors and other interested persons should read the registration statement and the Proxy Statement and other such documents carefully, when they become available, before making any voting or investment decisions. You may obtain free copies of these documents from the sources indicated above. No Offer or Solicitation This communication shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities in the Transaction shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Contacts Investor RelationsStephanie CarringtonICR HealthcareStephanie.Carrington@icrhealthcare.com(646) 277-1282 Media RelationsSean LeousICR HealthcareSean.Leous@icrhealthcare.com(646) 866-4012
Myant Acquires mmHg Inc. To Enhance Precision Medicine for Cardiovascular Care
TORONTO, Jan. 6, 2025 /PRNewswire/ — Myant Corp., a leader in chronic disease prevention through precision medicine, announced the acquisition of mmHg Inc., a digital health company specializing in remote blood pressure monitoring, cardiovascular risk reduction, and chronic disease…
Stroke Detection Innovator Wellumio Expands into the U.S. Market
Revolutionary portable device leverages advanced magnetic resonance technology to rapidly detect acute stroke biomarkers, enabling frontline care teams to make faster, life-saving decisions within the critical ‘golden hour.’ SAN FRANCISCO, Jan. 6, 2025 /PRNewswire/ — Wellumio, a New…
Viper Partners Drives Strategic Growth in Vascular Surgery Practices
PALM BEACH, Fla., Jan. 3, 2025 /PRNewswire/ — Viper Partners, a premier leader in healthcare investment banking, announces its continued commitment to the growth and advancement of vascular surgery practices nationwide. By facilitating strategic mergers and acquisitions, Viper Partners…
New HFSA Consensus Statement Provides Practical Guide for Implementing Palliative Care with Heart Failure Patients
WASHINGTON, Nov. 26, 2024 /PRNewswire/ — Patients with heart failure (HF) suffer from compromised quality of life, high mortality, and complex medical decision-making. Palliative care is an essential part of a comprehensive HF care plan. Integration of Palliative Care into Heart Failure…
Scriptor Software Unveils Free AI-Powered Software for Radiology Impressions
Revolutionizing Radiology Reporting with rScriptor Impressions PITTSBORO, N.C., Nov. 26, 2024 /PRNewswire/ — Scriptor Software is proud to announce the launch of rScriptor Impressions, a groundbreaking, free software tool that leverages Generative AI to automatically create radiology…