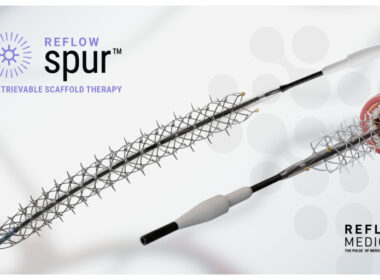
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc., a leading developer of innovative devices for treating complex cardiovascular disease, announces the completion of enrollment in the DEEPER CORONARY study. This pilot study evaluates the Spur® Elute Coronary Sirolimus-Eluting Retrievable Scaffold System as a primary treatment for in-stent restenosis (ISR) of the coronary arteries […]
Tag: ReFlow Medical
FDA Grants De Novo Clearance for Reflow Medical’s Spur® Peripheral Retrievable Stent System
San Clemente, CA – May 29, 2025 – Reflow Medical, Inc., a leading developer of innovative medical devices focused on complex cardiovascular disease, announced that the U.S. Food and Drug Administration (FDA) has granted De Novo clearance for the company’s Spur Peripheral Retrievable Stent System a unique clinical solution for the treatment of […]
Reflow Medical Enrolls First Patients in Pilot Study of Coronary Sirolimus-Eluting Retrievable Scaffold System (Spur Elute)
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc., a developer of innovative devices focused on cardiovascular disease, announces the first patient enrollments in “A pilot study of the Drug-Eluting Coronary Spur™ StEnt as a Primary trEatment for in-stent Restenosis (ISR) of the CORONARY arteries” (DEEPER CORONARY, NCT06117150). ISR is a common clinical problem that can generate significant […]
Reflow Medical Completes Enrollment in the DEEPER REVEAL Clinical Trial
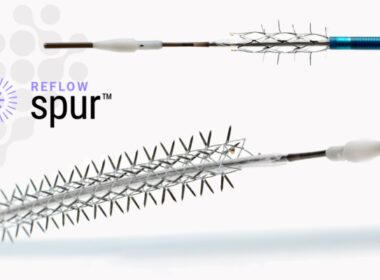
May 09, 2024 07:00 AM Eastern Daylight Time SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc., a developer of innovative devices focused on cardiovascular disease, announces completion of enrollment in the DEEPER REVEAL clinical trial (NCT05358353) to evaluate the Reflow Spur™ Stent. Designated a Breakthrough Device by the FDA, the Spur is a […]
Reflow Medical Receives CE Mark for Bare Temporary Spur Stent System for Treating de novo or Restenotic Below-the-Knee (BTK) Lesions
San Clemente, CA—Jan. 15, 2024 – Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System. The device is intended to treat de novo or restenotic lesions […]
Reflow Medical Introduces the coraCatheters™ Line and Expands into Complex Percutaneous Coronary Interventions (PCI)
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc. announced that it has received FDA commercial clearance for its coraCatheters™, a complete line of state-of-the-art microcatheters engineered to access and cross complex and challenging lesions in percutaneous coronary interventions. The coraCatheters family of novel devices is the latest in the Reflow portfolio, which […]
First Patient Enrolled in Reflow Medical’s DEEPER REVEAL IDE Clinical Study
San Clemente, CA—Reflow Medical, Inc., a medical device company focused on cardiovascular disease announces that the first patient was successfully treated and enrolled in the DEEPER REVEAL investigational device exemption (IDE) clinical trial (NCT05358353) at Advanced Cardiac & Vascular Centers (ACV) in Grand Rapids, Michigan. The Bare Temporary Spur Stent […]
Reflow Medical Completes DEEPER LIMUS Clinical Trial Enrollment
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc., a California-based medical device company, has completed enrollment in the DEEPER LIMUS clinical trial (NCT04162418) to evaluate the Temporary Spur Stent System, a patented device designed to treat long, diffuse and severely calcified infrapopliteal disease. The system allows for uniform expansion of the stent […]
Enrollment Completed in Reflow Medical’s DEEPER OUS Clinical Trial
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc. announces that it has completed patient enrollment in the DEEPER OUS clinical trial (NCT03807531) for the company’s Temporary Spur Stent System, a novel device that features a retrievable stent designed for complex infrapopliteal disease. 106 patients have now been enrolled in the prospective, nonrandomized […]
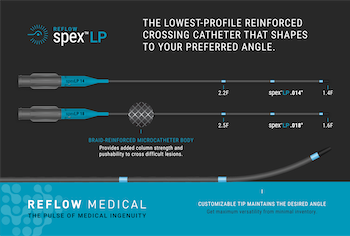
Reflow Medical Introduces the Spex™ LP, the Lowest Profile Shapeable Reinforced Support Catheter
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc., a California-based medical device company, introduces the Reflow™ Spex™ LP (Low Profile) 0.014 and 0.018-inch reinforced support catheters. The new Spex LP is engineered to provide the lowest profile tip for accessing and crossing the tightest and most complex lesions with a supportive system. It also […]