CONFIRM2 Registry Demonstrates Prognostic Value of AI-Based Plaque Characterization Beyond Stenosis Severity October 27, 2025 (DENVER, CO) – Cleerly, a leader in AI-based cardiovascular imaging, presented new, late-breaking science from the international CONFIRM2 Registry at the Transcatheter Cardiovascular Therapeutics (TCT) 2025 Conference at the Moscone Center in San Francisco, […]
Coronary/Structural Heart
Landmark STORM-PE Randomized Controlled Trial Finds Computer Assisted Vacuum Thrombectomy (CAVT) with Anticoagulation Superior to Traditional Anticoagulation Treatment for Pulmonary Embolism
ALAMEDA, Calif. – Oct. 27, 2025 – Penumbra, Inc. (NYSE: PEN) announced the results of the landmark STORM-PE randomized controlled trial (RCT), which found that the use of mechanical thrombectomy, specifically computer assisted vacuum thrombectomy (CAVT™), with anticoagulation achieved superior reduction in right heart strain compared to anticoagulation therapy alone in […]
U.S. FDA Approves Updated Indication for WINREVAIR™ (sotatercept-csrk) in Adults with Pulmonary Arterial Hypertension (PAH, WHO* Group 1 Pulmonary Hypertension) Based on Phase 3 ZENITH Study
Adding WINREVAIR to background PAH therapy improved exercise capacity and WHO functional class (FC), and reduced the risk of clinical worsening events, including hospitalization for PAH, lung transplantation and death ZENITH data add to growing body of evidence supporting a positive benefit risk profile of WINREVAIR in a broad […]
Six-month SPYRAL AFFIRM results from Medtronic demonstrate blood pressure reductions in high-risk cardiovascular patients
Medtronic also announced completion of enrollment in SPYRAL AFFIRM, which aims to build robust real-world evidence across traditionally underserved patient populations Medtronic plc, a global leader in healthcare technology, today announced the completion of enrollment and the first results from the High Cardiovascular Risk Patient Cohort of the SPYRAL AFFIRM […]
AtriCure Announces the First Patient Treated in the BoxX-NoAF Clinical Trial
Trial will evaluate the safety and effectiveness of the AtriCure Isolator® Synergy™ EnCompass® clamp and AtriClip® Left Atrial Appendage Exclusion System to reduce the occurrence of new-onset atrial fibrillation in cardiac surgery patients MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in surgical treatments and therapies for atrial fibrillation (Afib), left atrial appendage […]
Edwards SAPIEN 3 TAVR Delivers Proven Long-term Benefits and Valve Performance, New Data Presented at TCT 2025
SAPIEN 3, which showed superiority at 1 year, also demonstrates compelling outcomes equivalent to surgery at seven years SAN FRANCISCO–(BUSINESS WIRE)–Edwards Lifesciences (NYSE: EW) today announced seven-year data from the PARTNER 3 trial, reaffirming the early and sustained patient benefits of Edwards TAVR. The data, which showed superior clinical outcomes […]
Cardiosense Presents New Clinical Analysis of SEISMIC-HF I Data on AI-Enabled Volume Assessment in Heart Failure at the Transcatheter Cardiovascular Therapeutics (TCT) 2025 Scientific Symposium
CHICAGO–(BUSINESS WIRE)–Cardiosense, a digital health company pioneering AI-enabled solutions for cardiac disease management, today announced a new subgroup analysis of clinical data from the SEISMIC-HF I study at the Cardiovascular Research Foundation’s 2025 annual Transcatheter Cardiovascular Therapeutics® (TCT®) scientific symposium. The analysis examined the prescribed medications for symptomatic patients hospitalized with […]
Anteris Technologies Announces First Patients Treated in DurAVR® THV Global Pivotal Trial (the “PARADIGM Trial”)
MINNEAPOLIS, United States and BRISBANE, Australia, Oct. 27, 2025 (GLOBE NEWSWIRE) — Anteris Technologies Global Corp. (Anteris or the Company) (NASDAQ: AVR, ASX: AVR) a global structural heart company committed to designing, developing, and commercializing cutting-edge medical devices to restore healthy heart function, today announced the first patients have been enrolled and successfully treated in the DurAVR® Transcatheter Heart Valve (THV) global pivotal trial for patients with severe calcific aortic stenosis (the “PARADIGM Trial”). The procedures were performed by Prof. Dr. Ole De Backer at, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark. “We are proud to be the first enrolling center for this important trial,“ said Prof. Dr. De Backer. “Our initial experience with the DurAVR® THV System has been very positive, and we look forward to providing definitive comparative evidence which could transform patient care.” “With the first patients now randomized in the PARADIGM Trial, we are actively generating the clinical evidence required to advance the DurAVR® THV toward commercialisation, expanding treatment options for aortic stenosis patients,” said Anteris Chief Medical Officer, Chris Meduri, M.D. “This head-to-head study will provide robust comparative evidence across all surgical risk groups, which we believe will differentiate our platform based on efficacy, safety and ease of use.” The PARADIGM Trial builds on Anteris’ existing clinical data set of 130 patients successfully treated with the DurAVR® THV, including de novo (first time) aortic stenosis cases, valve-in-valve (ViV) patients and complex anatomies such as bicuspid aortic valve patients. Anteris aims to drive the global PARADIGM Trial through the addition of further countries and sites in the near term, with planned expansion across the United States, Europe and Canada. Management believes strong enthusiasm from investigators is expected to translate into efficient recruitment and timely study advancement. About the PARADIGM Trial The PARADIGM Trial is a prospective randomized controlled trial* (RCT) which will evaluate the safety and effectiveness of the DurAVR® THV compared to commercially available transcatheter aortic valve replacements (TAVRs). This head-to-head study will enroll approximately 1,000 patients in the ‘All Comers Randomized Cohort’ with 1:1 randomization of patients who will receive either the DurAVR® THV or TAVR using commercially available and approved THVs. The PARADIGM Trial will assess non-inferiority on a primary composite endpoint of all-cause mortality, all stroke and cardiovascular hospitalization at one year post procedure. For further information, please refer to ClinicalTrials.gov (ClinicaTrials.gov ID NCT07194265). The planned expansion across other geographies includes additional cohorts. *A Premarket Approval (PMA) application requires a high level of clinical evidence to demonstrate reasonable assurance of safety and effectiveness for the intended use. Randomized controlled trials are generally considered Level 1 evidence, the highest level for determining the effectiveness of interventions in evidence-based medicine given RCTs mimimize bias and allow a clear comparison between treatment groups. About Anteris Anteris Technologies Global Corp. (NASDAQ: AVR, ASX: AVR) is a global structural heart company committed to designing, developing, and commercializing cutting-edge medical devices to restore healthy heart function. Founded in Australia, with a significant presence in Minneapolis, USA, Anteris is a science-driven company with an experienced team of multidisciplinary professionals delivering restorative solutions to structural heart disease patients. Anteris’ lead product, the DurAVR® Transcatheter Heart Valve (THV), was designed in partnership with the world’s leading interventional cardiologists and cardiac surgeons to treat aortic stenosis – a potentially life-threatening condition resulting from the narrowing of the aortic valve. The balloon-expandable DurAVR® THV is the first biomimetic valve, which is shaped to mimic the performance of a healthy human aortic valve and aims to replicate normal aortic blood flow. DurAVR® THV is made using a single piece of molded ADAPT® tissue, Anteris’ patented anti-calcification tissue technology. ADAPT® tissue, which is FDA-cleared, has been used clinically for over 10 years and distributed for use in over 55,000 patients worldwide. The DurAVR® THV System is comprised of the DurAVR® valve, the ADAPT® tissue, and the balloon-expandable ComASUR® Delivery System. Forward-Looking Statements This announcement contains forward-looking statements, including statements regarding the planned expansion of the PARADIGM Trial, the results of the PARADIGM Trial, the quotes from Prof. Dr. De Backer and Chris Meduri, M.D., the contours of the PARADIGM Trial, and the expansion of the PARADIGM trial to other countries and cohorts. Forward-looking statements include all statements that are not historical facts. Forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “budget,” “target,” “aim,” “strategy,” “plan,” “guidance,” “outlook,” “may,” “should,” “could,” “will,” “would,” “will be,” “will continue,” “will likely result” and similar expressions, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described under “Risk Factors” in Anteris’ Annual Report on Form 10-K for the fiscal period ended December 31, 2024 that was filed with the SEC and ASX. Readers are cautioned not to put undue reliance on forward-looking statements, and except as required by law, Anteris does not assume any obligation to update any of these forward-looking statements to conform these statements to actual results or revised expectations. For more information: Investor Relations Investor Relations (US)investor@anteristech.com mchatterjee@bplifescience.comDebbie Ormsby Malini Chatterjee, Ph.D.Anteris Technologies Global Corp. Blueprint Life Science Group+61 1300 550 310 | +61 7 3152 3200 +1 917 330 4269 Website www.anteristech.com X @AnterisTech LinkedIn https://www.linkedin.com/company/anteristech
Elixir Medical Announces Significant Late Breaking Clinical Results for DynamX® Bioadaptor Demonstrating Nearly 50% Reduction in Coronary Event Rates vs. Current PCI Standard of Care
Results were presented at the Transcatheter Cardiovascular Therapeutics (TCT) Conference 2025 in San Francisco on October 27, 2025 DynamX® bioadaptor is the first interventional technology to demonstrate significant benefit with a reduction in long term adverse events in two consecutive randomized controlled trials MILPITAS, Calif., Oct. 27, 2025 (GLOBE NEWSWIRE) — Elixir Medical, a developer of disruptive technologies to treat cardiovascular disease, today announced new landmark clinical results for its DynamX® bioadaptor, a coronary implant designed to restore a blood vessel’s natural movement and function, also known as hemodynamic modulation, demonstrating a 48% risk reduction in device-related cardiac events compared to current-generation drug-eluting stent. The findings, presented on October 27th at the Transcatheter Cardiovascular Therapeutics (TCT) Conference 2025 in San Francisco, mark a major advance in coronary interventions after two decades of attempts to improve stenting outcomes. The results show that the DynamX® bioadaptor achieved a 48% reduction in Target Lesion Failure (TLF) endpoint – encompassing reductions in cardiac death, target vessel myocardial infarction, and ischemia-driven target lesion revascularization – from six months through two years (HR: 0.52 [0.29–0.93], p=0.027) in prespecified landmark superiority analysis. Additional findings include: Significant reduction in Target Vessel Failure (TVF) (p=0.048) from six months through two years in prespecified landmark analysis.Substantial clinical benefit for high-risk patients with Acute Coronary Syndrome (ACS) (p=0.018) from six months through two years in prespecified landmark analysis. “The INFINITY-SWEDEHEART long-term landmark results confirm our trial design hypothesis, demonstrating separation of the event curves for Target Lesion Failure (TLF) at six months and maintaining consistency up to two years with a reduction of TLF by 48%. Our data validates the unique property of the bioadaptor of restoring coronary physiology after unlocking after half a year, which translates into improved clinical outcomes long-term,” said Principal Investigator David Erlinge, M.D., Ph.D., Head of the Cardiology Department at Lund University, Lund, Sweden. The TCT presentation comes amid renewed global focus on rethinking coronary artery disease management. The recent Lancet Commission on rethinking coronary disease called for a paradigm shift from symptom-based treatment to earlier prevention and intervention, citing that cardiovascular disease affects roughly 19% of the global population and remains the world’s leading cause of death. In addition to delivering the DynamX® bioadaptor’s impressive results, presentations from multiple leading physicians investigating and demonstrating successful clinical outcomes with Elixir Medical technologies garnered a heavy podium presence each day at the TCT Conference. From October 25th through the 28th, investigators are unveiling new clinical and case performance data regarding the company’s LithiX™ Hertz Contact Intravascular Lithotripsy (HC-IVL) device while leading the discussion on topics like Is It Time to Move Beyond Stents for Lasting Patient Outcomes? and The Rebirth of Novel Technologies for Coronary PCI. As highlighted in a recent Wall Street Journal piece on physicians’ efforts to improve coronary artery disease treatment, in-stent restenosis and thrombosis after angioplasty (PCI) continue to drive repeat interventions and adverse outcomes despite widespread use of drug-eluting stents, adding to worsening long-term patient prognosis and significant healthcare costs. The DynamX® bioadaptor directly addresses these stent related issues by unlocking, adapting, and allowing the artery to heal and return to natural motion after fixated support is no longer needed: a key limitation of drug-eluting stents. About DynamX® Coronary Bioadaptor System The DynamX® bioadaptor is the first coronary implant technology designed to restore coronary artery hemodynamic modulation as demonstrated by restored vessel pulsatility, compliance, and adaptive increase in blood flow volume, and reduction in plaque progression. With its unique mechanism of action (MOA), it addresses the shortcomings of drug-eluting stents and bioresorbable scaffolds (BRS) with remarkably low clinical event rates that showed a plateau from six months through three-year clinical follow-up in BIOADAPTOR-RCT trial. The DynamX® Sirolimus Eluting Coronary Bioadaptor System is an investigational device in the United States limited by United States law to investigational use. The DynamX® Coronary Bioadaptor System is CE-mark approved. About Elixir Medical Elixir Medical Corporation, a privately held company based in Milpitas, California, develops disruptive platforms to treat coronary and peripheral artery disease. Our transformative technologies have multiple applications across the cardiovascular space capable of delivering improved clinical outcomes for millions of patients. Elixir Medical was named to Fast Company’s prestigious list of the World’s Most Innovative Companies of 2025 and Fierce Medtech’s 2025 Fierce 15 list. Visit us at www.elixirmedical.com and on LinkedIn and X. Media Contact Richard LaermerRLM PRelixir@rlmpr.com(212) 741-5106 X 216
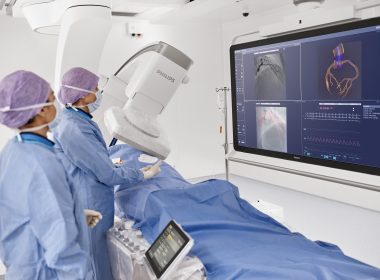
Philips introduces industry-first cath lab integration that automatically synchronizes pre-operative CT with C-arm movement, paving the way for CT-guided PCI
Philips Follow C-arm capability
Follow C-arm
October 27, 2025 New integration of Philips Advanced Visualization Workspace* with the Azurion image-guided therapy platform automatically synchronizes CT images with C-arm movement, supporting workflow efficiency and additional anatomical insights in PCI procedures1 San Francisco, USA and Amsterdam, The Netherlands – At the annual Transcatheter Cardiovascular Therapeutics (TCT 2025) meeting, Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today introduced an industry-first innovation that integrates pre-operative CT data directly into the cath lab workflow. This new capability, available through the integration of Philips’ Advanced Visualization Workspace (AVW) with the Azurion image-guided therapy system, marks a first step towards CT-guided percutaneous coronary intervention (PCI), a minimally invasive procedure to open narrowed coronary arteries and restore blood flow to the heart.The new capability, Follow C-arm, automatically synchronizes the 3D reconstruction of coronary arteries with the movement of the Azurion C-arm. As the C-arm angulation changes, the CT volume rotates in real time to match, giving interventionalists the 3D anatomical view without manual interaction. This seamless connection helps clinicians combine the detailed insights of CT imaging with the flexibility of live X-ray guidance inside the cath lab. The combined AVW–Azurion approach aims to provide enhanced anatomical insights to guide complex PCI procedures, publications have shown that leveraging CCTA may lead to reduction in contrast medium use and radiation dose during interventions.1Supporting the shift towards CT-guided PCICoronary computed tomography angiography (CCTA) is increasingly used in global clinical guidelines as a first-line tool for the diagnosis and planning of coronary artery disease. With more patients now arriving at the cath lab with prior CT scans, physicians are seeking ways to incorporate this information into their interventional workflows. By integrating CT data directly into Azurion, Philips is helping interventionalists expand the use of CT beyond diagnosis and planning, supporting a future in which CT-guided PCI becomes standard practice.“By bringing pre-operative CT into the cath lab and linking it directly to the movement of the C-arm, Philips is delivering an industry-first that helps interventionalists prepare for and execute PCI procedures with greater confidence,” said Mark Stoffels, Business Leader Image-Guided Therapy Systems at Philips. “This seamless integration is a significant step towards CT-guided PCI, aligning with our commitment to improving workflow efficiency and advancing patient care in interventional cardiology.”The launch builds on the global success of Philips’ Azurion image-guided therapy platform, the world’s leading system designed for seamless integration of advanced applications. Since its introduction in 2017, Azurion has been used to treat more than 6.4 million patients annually in over 80 countries, helping physicians perform minimally invasive procedures with greater confidence and efficiency.For more information about Philips’ presence at TCT, please visit the Philips TCT 2025 landing page. For further information, please contact:Joost MalthaPhilips Global External RelationsTel.: +31 6 1055816E-mail: joost.maltha@philips.com About Royal Philips Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home.Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2024 sales of EUR 18 billion and employs approximately 67,800 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter. Disclaimer: * The Follow C-arm capability as part of Philips Advanced Visualization Workspace may not be available in all markets. Please contact your Philips representative for more details.Reference: 1. J Cardiovasc Comput Tomogr. 2025 May-Jun;19(3):277-290.
Attachments
Philips Follow C-arm capability
Follow C-arm