BOTHELL, WA, March 7, 2019 /PRNewswire/ — BTG plc (LSE: BTG), the global healthcare company, exhibited the BTG Crossing Devices portfolio at the Chronic Total Occlusion (CTO) Summit in New York City from February 28 to March 1. Dr. Alexandre Avran of the Arnault Tzanck Institute in Nice, France, headlined the BTG exhibition at the summit this year, discussing his experience […]
Coronary/Structural Heart
New Data From Amgen To Be Presented At ACC.19 Continues To Build Evidence For Repatha® (evolocumab) Across Multiple Patient Populations
THOUSAND OAKS, Calif., March 6, 2019 /PRNewswire/ — Amgen (NASDAQ: AMGN) today announced the presentation of nine cardiovascular scientific research abstracts, including safety and efficacy results from the largest and longest open label study of homozygous familial hypercholesterolemia (HoFH) patients (TAUSSIG),1 as well as a sub-analysis from the Repatha® (evolocumab) cardiovascular outcomes study (FOURIER). Additional abstracts […]
Late-Breaking Data Presentations at ACC 2019 Highlight Abbott’s Leading Cardiovascular Portfolio
ABBOTT PARK, Ill., March 6, 2019 /PRNewswire/ — Key medical devices across Abbott’s (NYSE: ABT) cardiovascular portfolio will be highlighted in five late-breaking clinical trials at the American College of Cardiology’s 68th Annual Scientific Session (ACC 2019) from March 16 – 18 in New Orleans. The late-breaking studies showcase the depth and breadth of the company’s cardiovascular portfolio, providing new data […]
Le Bonheur Children’s Hospital to expand Heart Institute
MEMPHIS, Tenn., March 5, 2019 /PRNewswire/ — Le Bonheur Children’s Hospital will expand its Heart Institute with a two-story, $37.6 million addition. This expansion will add 19 additional beds to the Heart Institute to create a 31-bed dedicated Cardiovascular Unit. The hospital will expand west to the corner of Poplar Avenue and Dunlap Street. “We […]
Water Street’s Partnership with Leading Medical Products Company Leads to FDA Approval and Launch of Ready-to-Use Cardiovascular Medicine Eptifibatide
CHICAGO, March 6, 2019 /PRNewswire/ — Water Street Healthcare Partners, a strategic investor focused exclusively on the health care industry, announced today that its development partnership with a leading medical products company has resulted in the U.S. Food and Drug Administration (FDA) approval and launch of the ready-to-use cardiovascular medication, eptifibatide. It is […]
FDA Grants Saranas De Novo Designation for the Early BirdTM Bleed Monitoring System
HOUSTON–(BUSINESS WIRE)–Saranas Inc., a medical device company with innovative technology for real-time detection and monitoring of internal bleeding during endovascular procedures, today announced that it has been granted de novo classification by the U.S. Food and Drug Administration (FDA) for the Early Bird Bleed Monitoring System. According to a recent […]
ObEN Creates Healthcare Avatar for MedStar Health Heart Disease Study
PASADENA, Calif, March 05, 2019 (GLOBE NEWSWIRE) — (via Blockchain Wire) ObEN Inc., the artificial intelligence (AI) company creating Personal AI (PAI) technology to revolutionize digital interactions, is pleased to announce it is working with MedStar Health Research Institute for a study on the use of Personal AI (PAI) healthcare assistants […]
Kinetic River Corp. is Selected by UC Davis to Collaborate on NSF Research Grant
Mountain View, Calif., March 5, 2019 /PRNewswire/ — Kinetic River Corp., a leader in custom flow cytometry instrumentation, announced today that it has been selected by the University of California, Davis, as a subcontractor on a National Science Foundation research grant. Prof. James Chan, the Principal Investigator on the NSF grant, is leading the […]
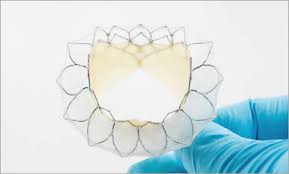
Neovasc’s Tiara™ Mitral Valve Replacement Technology Featured in Update Presentation at the CRT 2019 Meeting
VANCOUVER, March 4, 2019 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ: NVCN)(TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, today announced that its Tiara™ (“Tiara”) transcatheter mitral valve replacement device […]
VGS Completes Enrollment of VEST U.S. Pivotal Trial for the Evaluation of Its Venous External Support Technology for Coronary Bypass Surgery
TEL AVIV, Israel, March 5, 2019 /PRNewswire/ — Vascular Graft Solutions Ltd. (VGS) announces the completion of patient enrollment in the company’s US Investigational Device Exemption (IDE) randomized, controlled pivotal study, evaluating the efficacy of the VEST external support for saphenous vein grafts in coronary artery bypass (CABG) surgery. The study enrolled 224 […]