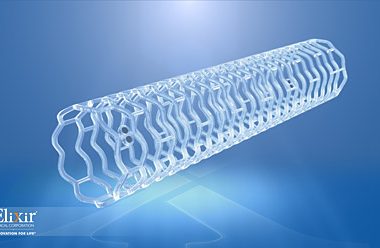
MILPITAS, Calif.–(BUSINESS WIRE)–Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling technologies designed to mimic the normal arterial function after cardiovascular and peripheral vascular disease intervention, announced today it will unveil a game-changing metallic drug eluting stent (DES) platform at this year’s Transcatheter Cardiovascular Therapeutics (TCT) conference […]
Coronary/Structural Heart
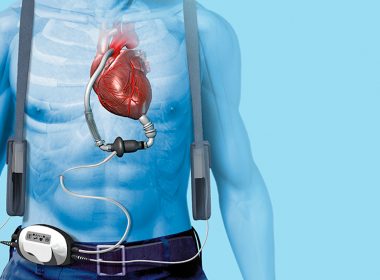
The Ethical Quandary of LVAD Disconnection
By Ken Dropiewski, Prime-Core Executive Search (ken@prime-core.com) New research brings to light a question of ethics when it comes to the deactivation of a left ventricular assist device (LVAD). Concerns differ significantly between cardiologists, hospice, and palliative medicine clinicians. This has lead to inconsistent and confusing end-of-life care for some […]
HeartStitch® GmbH Announces NobleStitch™ EL Closure Data Compared to Published PFO Clinical Trials “RESPECT” and “CLOSURE” in New England Journal of Medicine
FRANKFURT, Germany, Oct. 6, 2017 /PRNewswire/ — Prof. Dr. Achille Gaspardone, Director of Cardiology at Hospital of Sant’Eugenio (Rome, Italy) presented a comprehensive report on closure of PFO (Patent Foramen Ovale) utilizing the NobleStitch™ EL suture based closure system at the CSI-UCSF meeting on Congenital Structural Interventions in San Francisco. The NobleStitch™ EL, […]
BIOTRONIK Announces First Enrollments to BIOVITESSE Trial
(PresseBox) – BIOTRONIK has announced the start of enrollment of a coronary stent trial aiming at assessing the safety and clinical performance of a new coronary stent in de novo coronary artery lesions. On September 28, first Dr. Marco Moccetti, Cardiocentro Ticino, Lugano, Switzerland, and later on the same day Dr. Lorenz Raeber, University […]
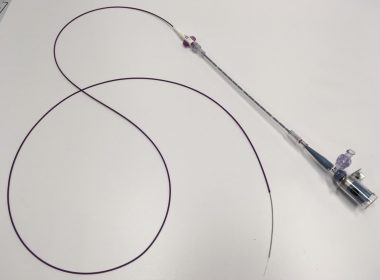
Essential Medical Release: The MANTA Large Bore Vascular Closure Device To Be Evaluated In A 500-Patient European Post Market Clinical Registry
EXTON, Pennsylvania, Oct. 9, 2017 /PRNewswire/ –Essential Medical, Inc. today announced initiation of enrollment in a post market clinical registry in the regions where MANTA is commercially available. Principle Investigator Nicolas Van Mieghem, MD, PhD, Medical Director of the Department of Interventional Cardiology at Thoraxcenter, Erasmus Medical Center, Rotterdam, Netherlands stated, “MANTA has quickly become […]
BioCardia Receives U.S. Patent Covering Morph Product Family Design
SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. (OTC: BCDA), a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, with clinical programs in heart failure, today announced the issuance of United States Patent No. 9,775,963 entitled, “Steerable Endoluminal Devices and Methods.” BioCardia CEO Peter Altman, PhD stated, “This new patent […]
BioVentrix Announces First Patient Enrolled In The U.S. Arm Of IDE Study Of The Revivent TC TransCatheter Ventricular Enhancement Treatment
SAN RAMON, Calif., and PITTSBURGH, Pa., Oct. 4, 2017 /PRNewswire/ — BioVentrix, Inc. a pioneer of technologies and procedures for less invasive treatment of heart failure (HF), today announced enrollment of the first patient in the U.S. arm of the ALIVE pivotal clinical trial. The trial is designed to demonstrate the safety and effectiveness […]
New cardiac catheter combines light and ultrasound to measure plaques
To win the battle against heart disease, cardiologists need better ways to identify the composition of plaque most likely to rupture and cause a heart attack. Angiography allows them to examine blood vessels for constricted regions by injecting them with a contrast agent before X-raying them. But because plaque does […]
Study Shows Significant Link Between AMI and This Common Illness
By Ken Dropiewski, Prime-Core Executive Search (ken@prime-core.com) Shingles are caused when the dormant Chickenpox virus reactivates. The result is an excruciatingly painful rash that causes inflammation of the underlying nerve tracks. This condition tends to develop at the most inopportune times such as during periods of stress and illness. It […]
Advanced Bifurcation Systems Announces Plan for Human Clinical Trials in New Brunswick, Canada
LOS ANGELES, Oct. 03, 2017 (GLOBE NEWSWIRE) — Advanced Bifurcation Systems (“ABS” or the “Company”), a clinical stage medical device company developing an innovative stenting platform which overcomes the limitations of current approaches for the treatment of bifurcation lesions in coronary angioplasties, today announced that the Company is planning to […]