NEWS PROVIDED BY Keystone Heart Ltd. Aug 01, 2017, 13:06 ET CAESAREA, Israel and TAMPA, Fla., Aug. 1, 2017 /PRNewswire/ — Keystone Heart Ltd., an emerging medical device company focused on developing cerebral protection devices for patients undergoing cardiac procedures, today announced plans to initiate clinical trials for a new, advanced version of Keystone Heart TriGuard™ cerebral […]
Coronary/Structural Heart
Want Half of Your Patients Who Smoke to Quit? Read This.
By Ken Dropiewski (ken@prime-core.com) Researchers in Denmark have once again proven the old adage that “seeing is believing.” The small study found when cardiac patients were shown images of their diseased arteries during follow-up, it influenced them to make significant and life-altering changes their diet and other habits. Want to […]
Boston Scientific Nixes Bioresorbable Stent Program
Boston Scientific decides it’s too risky and that there are “bigger” problems to deal with. Boston Scientific to end Renuvia bioresorbable coronary stent program JULY 31, 2017 BY FINK DENSFORD, MassDevice Boston Scientific (NYSE:BSX) is looking to terminate its Renuvia bioresorbable coronary stent program, according to a Minneapolis Star Tribune report. The move comes after […]
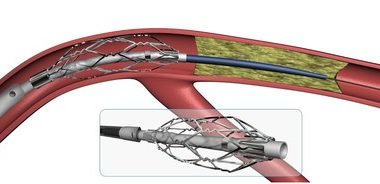
Medtronic Announces CE Mark and European Launch of CoreValve(TM) Evolut(TM) PRO Transcatheter Valve with Advanced Sealing
DUBLIN – July 31, 2017 – Medtronic plc (NYSE:MDT) today announced CE (Conformité Européenne) mark and European launch of the CoreValve(TM)Evolut(TM) PRO valve for the treatment of severe aortic stenosis for symptomatic patients who are at intermediate, high or extreme risk for open heart surgery. Clinical data for the Evolut PRO valve […]
BioCardia Completes Roll-In Cohort In Pivotal Phase III CardiAMP Heart Failure Trial
SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced completion of treatment for the 10-patient roll-in cohort for the pivotal Phase III CardiAMP Heart Failure Trial. A pre-specified review of the 30-day outcomes in this cohort by the Data […]
The Cath Lab of the Future is Here
JULY 26, 2017 BY Ken Dropiewski (ken@prime-core.com) In March, Miami Cardiac & Vascular Institute announced completion of its state-of-the-art cath lab, with the first next ten image-guided therapy system was being designed to reduce radiation exposure, decrease prep and procedure time and visually eliminate human error in the preparation and treatment […]
ABBOTT INITIATES CLINICAL TRIAL OF THREE-MONTH DUAL ANTIPLATELET THERAPY FOLLOWING IMPLANTATION WITH XIENCE CORONARY STENT
PRESS RELEASE ABBOTT PARK, Ill., July 25, 2017 — Abbott today announced that the first patient has been enrolled in a clinical study evaluating the short-term use of common blood thinning medicines, called dual antiplatelet therapy (DAPT), after receiving a XIENCE everolimus-eluting coronary stent. The study, called XIENCE Short DAPT, […]
Roxwood Medical Announces Agreement With Abbott (ABT) For Distribution Of Products In U.S.
REDWOOD CITY, Calif., July 25, 2017 /PRNewswire/ — Roxwood Medical Inc., a leading provider of advanced cardiovascular specialty catheters, today announced it has entered into an exclusive agreement with Abbott for distribution of Roxwood products in the United States. “This partnership with Abbott’s vascular business is a major milestone for Roxwood that allows far […]
AAP counsels pediatricians to focus on clusters of cardiometabolic risk factors to help obese kids
WASHINGTON, July 24, 2017 /PRNewswire-USNewswire/ — Because obesity affects one in six U.S. children and adolescents, there is a pressing need to identify the subset of overweight or obese kids at the highest risk of developing cardiovascular and metabolic complications and to direct interventions to them. Since frameworks used to identify adults […]
4C Medical rakes in $9M
TMVR dev 4C Medical raises $9m JULY 20, 2017 BY FINK DENSFORD – MassDevice Structural heart disease device maker 4C Medical said today it completed a private placement of unsecured convertible promissory notes, raising approximately $9 million to help support development of its transcatheter tech designed to treat mitral regurgitation. The financing was led by […]